Bipyridyl palladium embedded porous organic polymer as highly efficient and reusable heterogeneous catalyst for Suzuki–Miyaura coupling reaction†
Chang-An Wang*,
Yin-Feng Han,
Yan-Wei Li,
Kun Nie,
Xue-Li Cheng and
Jian-Ping Zhang
College of Chemistry and Chemical Engineering, Taishan University, Tai'an, Shandong 271000, P. R. China. E-mail: wangcha_chem@163.com
First published on 4th April 2016
Abstract
Two robust porous organic polymers embedded with bipyridyl ligand (Bpy-POP) were obtained via a bottom-up strategy. After a simple post-treatment, a Pd(II)-containing POP (Bpy-Pd-POP) was used as highly efficient and reusable heterogeneous catalyst for the Suzuki–Miyaura coupling reaction. It could be reused at least 15 times without significant loss of the catalytic activity (97–99% yield).
Chelating ligands containing pyridyl moieties are extensively used ligand frameworks in coordination chemistry1 and homogeneous catalysis.2 As one of the most important chelating ligands, the bipyridine has offered an interesting alternative in developing catalytic systems for organic synthesis due to its robust redox stability, coordinating ability with a wide range of metal ions and ease of functionalization.2a,b,e However, many noble metals used in homogeneous catalysis are quite expensive and it is difficult to separate the ligand–metal catalysts from the reaction system, which restricted their practical application on a large scale. Accordingly, immobilization of homogeneous catalysts using a solid support provide an interesting approach to solve these vital problems.3
In this context, porous organic polymers (POPs),4 due to their large surface area, low skeletal density and high chemical stability, have attracted particular attention as a new class of porous materials for various potential applications.5–9 In particular, POPs have provided a highly tunable platform for designing heterogeneous catalysts via the embeddedness of rigid catalytic active monomers into the frameworks.10 Importantly, the bipyridine ligand coordinated with metal ions embedded POPs framework was shown high catalytic activity for heterogeneous catalysis. For example, Lin and co-workers developed the synthesis of phosphorescent [Ru(bpy)3]2+ and [Ir(ppy)2(bpy)]+-incorporating porous cross-linked polymers for efficient photocatalysis.11 Cooper and co-workers elaborated the synthesis of a series of the bipyridine metal-functionalized conjugated microporous polymers as an efficient heterogeneous catalyst for reductive amination.12 However, to the best of our knowledge, the recyclable and reusable (<5 times) of these bipyridine metal-functionalized POPs are not satisfactory. Therefore, towards the practical applications of POP catalysts, the following criteria should be considered: (i) the synthesis of POP catalysts should be concise and based on inexpensive chemicals; (ii) the POP catalysts should be robust and has high activity and selectivity; (iii) the POP catalysts should possess persistent recycling and reusing.
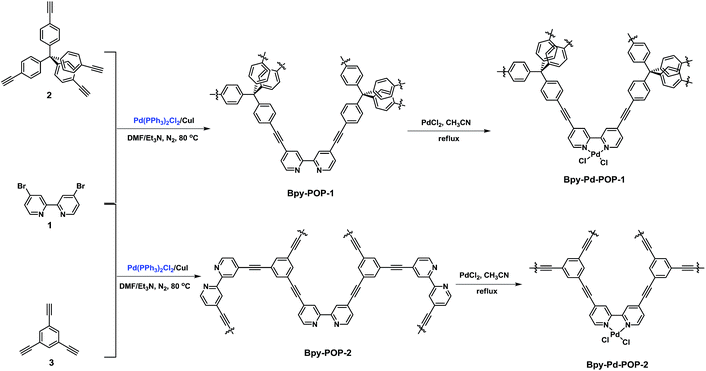
With these considerations in mind, herein, we report the synthesis of two robust porous organic polymers embedded with bipyridyl ligand (Bpy-POP) via a bottom-up strategy. After being post-modified with palladium ions, these polymers (Bpy-Pd-POP, Scheme 1) could be utilized as highly efficient and recyclable heterogeneous catalysts for carbon–carbon coupling reaction. Inspired by the pioneering work of Cooper and co-workers, where the surface area and the pore size of CMPs could be tuned by varying the molecular length of the rigid building blocks (alkynes).13 Accordingly, we could synthesize two robust bipyridyl-functionalized POPs with tunable surface areas and pore size distributions, and preliminary investigate the influence of the properties on the catalytic activity.
The polymer networks were constructed by palladium-catalyzed Sonogashira–Hagihara cross-coupling reaction of 4,4′-dibromo-2,2′-bipyridine (1) as the functional building blocks with tetra(4-ethynylphenyl)methane (2) or 1,3,5-triethynylbenzene (3) as the structural building blocks (see the ESI for Experimental details†). The general synthetic routes for the Bpy-POP networks are shown in Scheme 1. Both of the obtained polymers Bpy-POP-1 and Bpy-POP-2 are a dark brown powder, and are insoluble in all of the solvents tested. The polymer is thermally stable up to 400 °C under nitrogen atmosphere (TGA result for Bpy-POP shown in Fig. S1 and S2 in the ESI†). Powder X-ray diffraction measurements indicated that the Bpy-POP network is amorphous in nature (Fig. S3, ESI†).
The permanent porosity of Bpy-POP was investigated by nitrogen sorption measurements at 77 K, and the obtained results are listed in Table S1.† The application of the Brunauer–Emmett–Teller (BET) modal resulted in the surface areas of 506 and 443 m2 g−1 for Bpy-POP-1 (Fig. S4, ESI†) and Bpy-POP-2 (Fig. S5, ESI†), respectively. By comparing Bpy-POP-1 (monomer 2) and Bpy-POP-2 (monomer 3), we found that the polymer networks with long-lengthed building block had a higher BET surface area (506 m2 g−1 vs. 443 m2 g−1). In Fig. 1(a) and (b) are shown the N2 volume adsorbed, Vads, vs. relative pressure P/Po, for Bpy-POP-1 and Bpy-POP-2. According to the IUPAC classification, POP-1 displays a type-IV gas sorption isotherm with H2 hysteresis loops, indicating that this polymer consist of mesoporous and POP-2 displays a type-I gas sorption isotherm reflecting the presence of abundant micropores structure. From t-plot analysis, the Bpy-POP-1 polymer displays both a microporous contribution of 206 m2 g−1 (40.7%) and a mesoporous contribution of 300 m2 g−1 (59.3%). As calculated by non-local density functional theory (NLDFT), the pore size distribution (PSD) of Bpy-POP-1 and Bpy-POP-2 mainly distributed around 0.7 and 1.3 nm, respectively (Fig. 1(c) and (d)). However, the broad PSD curves of POP-1 also suggest the co-existence of micro- and mesopores in the polymer networks. Specifically, the constructed mesopores greatly facilitate the mass transport process. After being post-modified with PdCl2, the BET surface area of Bpy-POP-1 decreases to 394 m2 g−1 due to the PdCl2 hanging in the pore volume.
 | ||
| Fig. 1 Nitrogen sorption isotherm of Bpy-POP-1 (a) and Bpy-POP-2 (b). Pore size distribution (PSD) for Bpy-POP-1 (c) and Bpy-POP-2 (d), calculated with non-local density functional theory (NLDFT). | ||
The chemical composition of Bpy-POP were characterized by solid-state 13C cross-polarization magic-angle spinning (CP/MAS) NMR spectroscopy. Fig. 2 shows the 13C CP/MAS NMR spectra recorded for Bpy-POP-1. The signals at δ = 121, 149 and 155 ppm confirmed that the bipyridyl monomer skeleton has well been embedded into the polymer networks, while the peak at approximately 88 and 93 ppm represent the alkynyl groups in the polymers. The peak at about 65, 131 and 149 ppm also confirm that the tetra(4-ethynylphenyl)methane skeleton has been successful embedded into the frameworks. Additionally, elemental analysis of Bpy-POP polymer identified the content of nitrogen (6.59% and 8.00%), based on which the molar loading of bipyridyl ligand moieties could be calculated. All of the results proved the successful synthesis of the polymer networks.
 | ||
| Fig. 2 Solid-state 13C CP/MAS NMR spectra for Bpy-POP-1. The assignments for the 13C MAS NMR signals are indicated (top). 13C CP/MAS NMR spectra for Bpy-POP-2 are shown in Fig. S6.† | ||
Next, through a simple post-treatment of Bpy-POP with palladium chloride (Scheme 1), the porous organic catalyst Bpy-Pd-POP was facilely prepared (see ESI for details†). We then performed X-ray photoelectron spectroscopy (XPS) measurements to investigate the incorporation of palladium within Bpy-Pd-POP-1. As shown in Fig. 3 (red), the binding energy (BE) at 336.88 eV correspond to Pd3d5/2 orbital in Bpy-Pd-POP-1, which indicated that the Pd species is present in +2 state.14 The BE of 336.88 eV for the Pd species in Bpy-Pd-POP-1 shifted negatively 0.75 eV in comparison with that of 337.63 eV for free PdCl2 (Fig. 3, black), which indicates the strong coordination of PdCl2 with the bipyridyl ligand of Bpy-POP-1 polymer. In addition, the slight difference in the FT-IR spectra of Bpy-POP and Bpy-Pd-POP also indicates that the coordinated palladium chloride in the polymer (Fig. S7 and S8, ESI†).
The Suzuki–Miyaura coupling reaction is one of the most versatile methods for the facile formation of C–C bonds, which has attracted extensive attention in recent years.15 To evaluate the catalytic activity of Bpy-Pd-POP as a robust heterogeneous catalyst, we accordingly chose the Suzuki–Miyaura coupling reaction of phenylboronic acid with bromobenzene as a model reaction (Table 1). Using Bpy-Pd-POP-1 polymer as the catalyst, the reaction occurred smoothly in EtOH at 80 °C to give the desired product with high yield (Table 1, entry 1). Further investigation on the effect of reaction media was conducted (Table 1, entries 2–6) and mixture solvent of EtOH and H2O with a ratio of 1/1 gave the best result (Table 1, entry 6, 99% yield). The effect of temperature on this reaction was also examined (Table 1, entries 7 and 8), when the temperature was lower to 60 °C, the reaction proceeded much slower in EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and a product yield of 95% could be achieved in more than 2 h (Table 1, entry 7). We hoped to investigate the influence of the properties on the catalytic activity, unfortunately, the Bpy-Pd-POP-2 polymer showed similar reactivity to Bpy-Pd-POP-1 under this system (Table 1, entry 9). Finally, the optimized condition for the Suzuki–Miyaura coupling reaction was chosen as: EtOH/H2O (1
1, v/v) and a product yield of 95% could be achieved in more than 2 h (Table 1, entry 7). We hoped to investigate the influence of the properties on the catalytic activity, unfortunately, the Bpy-Pd-POP-2 polymer showed similar reactivity to Bpy-Pd-POP-1 under this system (Table 1, entry 9). Finally, the optimized condition for the Suzuki–Miyaura coupling reaction was chosen as: EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as the co-solvent, 0.8 mol% Bpy-Pd-POP-1 as the heterogeneous catalyst, in the presence of potassium carbonate, and at 80 °C.
1, v/v) as the co-solvent, 0.8 mol% Bpy-Pd-POP-1 as the heterogeneous catalyst, in the presence of potassium carbonate, and at 80 °C.
| Entry | Catalyst | Solvent | Temp. (°C) | Time (h) | Yieldb (%) | TONc |
|---|---|---|---|---|---|---|
| a General condition: phenylboronic acid (0.5 mmol), bromobenzene (1.0 mmol), K2CO3 (1.0 mmol), and catalyst (0.8 mol%), solvent (1.0 mL), temperature.b Isolated yield after silica gel column chromatography.c TON = (moles of product)/(moles of metal in the catalyst), unless otherwise noted. | ||||||
| 1 | Bpy-Pd-POP-1 | EtOH | 80 | 1 | 95 | 119 |
| 2 | Bpy-Pd-POP-1 | H2O | 80 | 1 | 85 | 106 |
| 3 | Bpy-Pd-POP-1 | Toluene | 80 | 3 | 60 | 75 |
| 4 | Bpy-Pd-POP-1 | CH2Cl2 | 80 | 3 | 52 | 65 |
| 5 | Bpy-Pd-POP-1 | DMF | 80 | 3 | 60 | 75 |
| 6 | Bpy-Pd-POP-1 | EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 0.5 | 99 | 124 |
| 7 | Bpy-Pd-POP-1 | EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
60 | 2 | 95 | 119 |
| 8 | Bpy-Pd-POP-1 | EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
100 | 0.5 | 99 | 124 |
| 9 | Bpy-Pd-POP-2 | EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 0.5 | 98 | 123 |
| 10 | — | EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 3 | n.d. | 0 |
Having established the optimized reaction conditions, we tested a variety of substituted aryl halides to probe the versatility of our catalytic system (Table 2). As shown in Table 2, good to excellent isolated yields (93–99%) were obtained for all of the Suzuki–Miyaura coupling reaction of a variety of bromo and iodo derivatives with phenylboronic acid catalyzed by the Bpy-Pd-POP-1 catalyst (Table 2, entries 1–9). For aryl iodides, the coupling reactions were rapidly completed within 0.5 h, giving the corresponding products with high yield (Table 2, entries 8 and 9). The phenylboronic acid substrate with an electron-withdrawing substituent could also be converted into the corresponding adduct in high yield (Table 2, entry 10). These results identified that the Bpy-Pd-POP-1 nanoporous polymer works as a highly efficient heterogeneous catalyst for the Suzuki–Miyaura coupling reaction.
| Entry | Ar–X | Ar–B(OH)2 | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a General condition: phenylboronic acid (0.75 mmol), arylhalide (0.5 mmol), K2CO3 (1.0 mmol), and Bpy-Pd-POP-1 (0.8 mol%), EtOH/H2O (1.0 mL), 80 °C.b Isolated yield after silica gel column chromatography. | ||||
| 1 |  |
 |
0.5 | 99 |
| 2 |  |
 |
1.5 | 93 |
| 3 | 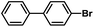 |
 |
7 | 99 |
| 4 |  |
 |
2 | 98 |
| 5 | 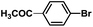 |
 |
2 | 98 |
| 6 | 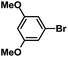 |
 |
1 | 99 |
| 7 |  |
 |
3 | 99 |
| 8 |  |
 |
0.5 | 99 |
| 9 | 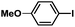 |
 |
0.5 | 94 |
| 10 |  |
 |
2 | 99 |
Next, we examined the recyclability of this porous organic catalyst Bpy-Pd-POP-1 (see ESI, Table S2†).
The recycling experiment was performed by using a simple centrifugation method to recover the heterogeneous catalyst. The recovered polymer was washed with EtOH and EtOAc to remove the residual product, and simple dried in vacuum overnight before reuse. As shown in Fig. 4, owing to the built-in character of the covalently linked catalytic sites in the polymer networks, the Bpy-Pd-POP-1 nanoporous catalyst can be reused at least fifteen times without any significant loss of the catalytic activity (97–99% yield).16 To determine that the reaction between bromobenzene and phenylboronic acid was indeed catalyzed by Bpy-Pd-POP-1 instead of the dissolved homogeneous Pd species leached from the supports, the following strategy was adopted. When the phenylboronic acid conversion reached about 50%, the solid catalyst was removed from the hot reaction mixture and then the mother liquor was allowed to reaction another 2 h under the same conditions.10f,17 No significant change in conversion or yield could be observed. This study identified that PdCl2 is strongly coordinated within Bpy-POP polymer and the active phase was not the dissolved Pd species leached from the polymer. In addition, XPS characterization of Bpy-Pd-POP-1 after catalytic runs showed the well-retained state of Bpy-Pd(II) catalytic species (Fig. S9, ESI†) and TEM images showed no Pd(0) nanoparticles were appeared in the frameworks of the recycled catalyst (Fig. S10, ESI†).
 | ||
| Fig. 4 Recycling experiment of Bpy-Pd-POP-1 for the Suzuki–Miyaura coupling reaction of bromobenzene and phenylboronic acid. | ||
To summarize, we have reported a powerful strategy to prepare Pd(II) organometallic catalyst immobilized on the bipyridyl ligand-functionalized porous organic polymer (Bpy-POP). Owing to the high BET surface area, covalently linked catalytic sites, wide opening and interconnected pores, the Bpy-Pd-POP displays high efficient activity and excellent recyclability for the Suzuki–Miyaura coupling reaction. The catalyst could be reused at least 15 times without significant loss of the catalytic activity (97–99% yield). We believe that Bpy-Pd-POP may act as a promising heterogeneous catalyst applicable to a variety of carbon–carbon coupling reactions, the topic of which is under further research in our laboratory.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21502136, 21402138, 21201131), the Promotive Research Fund for Excellent Young and Middle-aged Scientists of Shandong Province (No. BS2014CL035) and Higher Educational Science and Technology Program of Shandong Province (No. J15LC18).Notes and references
- (a) E. C. Constable and P. J. Steel, Coord. Chem. Rev., 1989, 93, 205 CrossRef CAS; (b) C. Kaes, A. Katz and M. W. Hosseini, Chem. Rev., 2000, 100, 3553 CrossRef CAS PubMed; (c) E. D. Bloch, D. Britt, C. Lee, C. J. Doonan, F. J. Uribe-Romo, H. Furukawa, J. R. Long and O. M. Yaghi, J. Am. Chem. Soc., 2010, 132, 14382 CrossRef CAS PubMed.
- (a) G. Chelucci and R. P. Thummel, Chem. Rev., 2002, 102, 3129 CrossRef CAS PubMed; (b) G. R. Newkome, A. K. Patri, E. Holder and U. S. Schubert, Eur. J. Org. Chem., 2004, 2004, 235 CrossRef; (c) M. Ye, G.-L. Gao, A. J. F. Edmunds, P. A. Worthington, J. A. Morris and J.-Q. Yu, J. Am. Chem. Soc., 2011, 133, 19090 CrossRef CAS PubMed; (d) M. Ye, G.-L. Gao and J.-Q. Yu, J. Am. Chem. Soc., 2011, 133, 6964 CrossRef CAS PubMed; (e) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed.
- For selected examples of immobilization of metal-bipyridyl catalysts, see: (a) G. A. Slough, V. Krchňák, P. Helquist and S. M. Canham, Org. Lett., 2004, 6, 2909 CrossRef CAS PubMed; (b) T. Osako and Y. Uozumi, Chem. Lett., 2009, 38, 902 CrossRef CAS; (c) T. Osako and Y. Uozumi, Heterocycles, 2010, 80, 505 CrossRef CAS; (d) J. Huang, W. Wang and H. Li, ACS Catal., 2013, 3, 1526 CrossRef CAS; (e) K. Manna, T. Zhang and W. Lin, J. Am. Chem. Soc., 2014, 136, 6566 CrossRef CAS PubMed.
- For selected reviews of POPs, see: (a) N. B. McKeown and P. M. Budd, Chem. Soc. Rev., 2006, 35, 675 RSC; (b) A. I. Cooper, Adv. Mater., 2009, 21, 1291 CrossRef CAS; (c) N. B. McKeown and P. M. Budd, Macromolecules, 2010, 43, 5163 CrossRef CAS; (d) X. Feng, X. Ding and D. Jiang, Chem. Soc. Rev., 2012, 41, 6010 RSC; (e) S.-Y. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548 RSC.
- For selected examples of POPs on gas storage, see: (a) J.-X. Jiang, F. Su, A. Trewin, C. D. Wood, N. L. Campbell, H. Niu, C. Dickinson, A. Y. Ganin, M. J. Rosseinsky, Y. Z. Khimyak and A. I. Cooper, Angew. Chem., Int. Ed., 2007, 46, 8574 CrossRef CAS PubMed; (b) T. Ben, H. Ren, S. Ma, D. Cao, J. Lan, X. Jing, W. Wang, J. Xu, F. Deng, J. M. Simmons, S. Qiu and G. Zhu, Angew. Chem., Int. Ed., 2009, 48, 9457 CrossRef CAS PubMed; (c) S. Yuan, S. Kirklin, B. Dorney, D.-J. Liu and L. Yu, Macromolecules, 2009, 42, 1554 CrossRef CAS; (d) E. Klontzas, E. Tylianakis and G. E. Froudakis, Nano Lett., 2010, 10, 452 CrossRef CAS PubMed; (e) A. Li, R.-F. Lu, Y. Wang, X. Wang, K.-L. Han and W.-Q. Deng, Angew. Chem., Int. Ed., 2010, 49, 3330 CrossRef CAS PubMed.
- For selected examples of POPs on gas separation, see: (a) O. K. Farha, A. M. Spokoyny, B. G. Hauser, Y.-S. Bae, S. E. Brown, R. Q. Snurr, C. A. Mirkin and J. T. Hupp, Chem. Mater., 2009, 21, 3033 CrossRef CAS; (b) Y. Luo, B. Li, W. Wang, K. Wu and B. Tan, Adv. Mater., 2012, 24, 5703 CrossRef CAS PubMed; (c) Q. Chen, M. Luo, P. Hammershøj, D. Zhou, Y. Han, B. W. Laursen, C.-G. Yan and B.-H. Han, J. Am. Chem. Soc., 2012, 134, 6084 CrossRef CAS PubMed; (d) L.-H. Xie and M. P. Suh, Chem.–Eur. J., 2013, 19, 11590 CrossRef CAS PubMed.
- For selected examples of POPs on molecular recognition/sensors, see: (a) X. Liu, Y. Xu and D. Jiang, J. Am. Chem. Soc., 2012, 134, 8738 CrossRef CAS PubMed; (b) J. Liu, K.-K. Yee, K. K.-W. Lo, K. Y. Zhang, W.-P. To, C.-M. Che and Z. Xu, J. Am. Chem. Soc., 2014, 136, 2818 CrossRef CAS PubMed.
- For selected examples of POPs on light-harvesting, see: (a) J. Weber and A. Thomas, J. Am. Chem. Soc., 2008, 130, 6334 CrossRef CAS PubMed; (b) L. Chen, Y. Honsho, S. Seki and D. Jiang, J. Am. Chem. Soc., 2010, 132, 6742 CrossRef CAS PubMed; (c) Y. Xu, L. Chen, Z. Guo, A. Nagai and D. Jiang, J. Am. Chem. Soc., 2011, 133, 17622 CrossRef CAS PubMed.
- For selected reviews of POPs on heterogeneous catalysis, see: (a) P. Kaur, J. T. Hupp and S. T. Nguyen, ACS Catal., 2011, 1, 819 CrossRef CAS; (b) Y. Zhang and S. N. Riduan, Chem. Soc. Rev., 2012, 41, 2083 RSC; (c) M. Rose, ChemCatChem, 2014, 6, 1166 CAS; (d) C.-A. Wang and W. Wang, Acta Chim. Sin., 2015, 73, 498 CrossRef CAS.
- For selected examples of POPs on heterogeneous catalysis, see: (a) X. Du, Y. Sun, B. Tan, Q. Teng, X. Yao, C. Su and W. Wang, Chem. Commun., 2010, 46, 970 RSC; (b) L. Chen, Y. Yang and D. Jiang, J. Am. Chem. Soc., 2010, 132, 9138 CrossRef CAS PubMed; (c) C. A. Wang, Z. K. Zhang, T. Yue, Y. L. Sun, L. Wang, W. D. Wang, Y. Zhang, C. Liu and W. Wang, Chem.–Eur. J., 2012, 18, 6718 CrossRef CAS PubMed; (d) Y. Zhang, Y. Zhang, Y. L. Sun, X. Du, J. Y. Shi, W. D. Wang and W. Wang, Chem.–Eur. J., 2012, 18, 6328 CrossRef CAS PubMed; (e) D. S. Kundu, J. Schmidt, C. Bleschke, A. Thomas and S. Blechert, Angew. Chem., Int. Ed., 2012, 51, 5456 CrossRef CAS PubMed; (f) B. Li, Z. Guan, W. Wang, X. Yang, J. Hu, B. Tan and T. Li, Adv. Mater., 2012, 24, 3390 CrossRef CAS PubMed; (g) M. H. Weston, O. K. Farha, B. G. Hauser, J. T. Hupp and S. T. Nguyen, Chem. Mater., 2012, 24, 1292 CrossRef CAS; (h) H. Li, B. Xu, X. Liu, A. Sigen, C. He, H. Xia and Y. Mu, J. Mater. Chem. A, 2013, 1, 14108 RSC; (i) J.-X. Jiang, Y. Li, X. Wu, J. Xiao, D. J. Adams and A. I. Cooper, Macromolecules, 2013, 46, 8779 CrossRef CAS; (j) Y. Zhang, B. Li and S. Ma, Chem. Commun., 2014, 50, 8507 RSC; (k) W.-K. An, M.-Y. Han, C.-A. Wang, S.-M. Yu, Y. Zhang, S. Bai and W. Wang, Chem.–Eur. J., 2014, 20, 11019 CrossRef CAS PubMed.
- (a) Z. Xie, C. Wang, K. E. deKrafft and W. Lin, J. Am. Chem. Soc., 2011, 133, 2056 CrossRef CAS PubMed; (b) J.-L. Wang, C. Wang, K. E. deKrafft and W. Lin, ACS Catal., 2012, 2, 417 CrossRef CAS.
- J.-X. Jiang, C. Wang, A. Laybourn, T. Hasell, R. Clowes, Y. Z. Khimyak, J. Xiao, S. J. Higgins, D. J. Adams and A. I. Cooper, Angew. Chem., Int. Ed., 2011, 50, 1072 CrossRef CAS PubMed.
- J. X. Jiang, F. Su, A. Trewin, C. D. Wood, H. Niu, J. T. A. Jones, Y. Z. Khimyak and A. I. Cooper, J. Am. Chem. Soc., 2008, 130, 7710 CrossRef CAS PubMed.
- (a) S.-Y. Ding, J. Gao, Q. Wang, Y. Zhang, W.-G. Song, C.-Y. Su and W. Wang, J. Am. Chem. Soc., 2011, 133, 19816 CrossRef CAS PubMed; (b) C. A. Thomas, Photoelectron and Auger Spectroscopy, Plenum, New York, 1975 Search PubMed.
- For selected examples of recent literature in Suzuki–Miyaura coupling reaction, see: (a) S. Dutt, R. Kumar and P. F. Siril, RSC Adv., 2015, 5, 33786 RSC; (b) M. Gholinejad, M. Razeghi and C. Najera, RSC Adv., 2015, 5, 49568 RSC; (c) S. Pradhan and R. P. John, New J. Chem., 2015, 39, 5759 RSC; (d) S. Sumino, T. Ui and I. Ryu, Org. Chem. Front., 2015, 2, 1085 RSC; (e) H. Li, Y.-L. Zhong, C. Chen, A. E. Ferraro and D. Wang, Org. Lett., 2015, 17, 3616 CrossRef CAS PubMed; (f) E. F. Altenhofer and M. Harmata, J. Org. Chem., 2015, 80, 8168 CrossRef CAS PubMed; (g) Y. Li, F. Mao, T. Chen, Z. Zhou, Y. Wang and J. Huang, Adv. Synth. Catal., 2015, 357, 2827 CrossRef CAS; (h) N. Iranpoor, S. Rahimi and F. Panahi, RSC. Adv., 2016, 6, 3084 RSC.
- In the recycling experiments, a progressive increase in reaction time is needed (shown in ESI†), which could be due to the partial blocking of the polymeric nanopores. See for example: (a) X. Du, Y. Sun, B. Tan, Q. Teng, X. Yao, C. Su and W. Wang, Chem. Commun., 2010, 46, 970 RSC; (b) C. A. Wang, Z. K. Zhang, T. Yue, Y. L. Sun, L. Wang, W. D. Wang, Y. Zhang, C. Liu and W. Wang, Chem.–Eur. J., 2012, 18, 6718 CrossRef CAS PubMed.
- R. A. Sheldon, M. Wallau, I. W. C. E. Arends and U. Schuchardt, Acc. Chem. Res., 1998, 31, 485 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra03331h |
| This journal is © The Royal Society of Chemistry 2016 |