Aggregation-induced emission and reversible mechanochromic luminescence of carbazole-based triphenylacrylonitrile derivatives†
Yong Zhan*a,
Peng Gongb,
Peng Yanga,
Zhe Jina,
Ying Baoa,
Ying Lia and
Yongnan Xu*a
aKey Laboratory of Structure-Based Drug Design and Discovery, Shenyang Pharmaceutical University, Ministry of Education, Shenyang, 110016, P. R. China. E-mail: zhanyong2046@126.com; Tel: +86-024-23986449
bState Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, 130012, P. R. China
First published on 24th March 2016
Abstract
Novel carbazole-based triphenylacrylonitrile derivatives (Cz1-TPAN and Cz2-TPAN) have been carefully prepared via the Heck coupling reaction. Both compounds exhibited typical intramolecular change transfer (ICT) and aggregation-induced emission (AIE) characteristics with high solid-state efficiency. For example, the ΦF of Cz1-TPAN in as-synthesized crystals reached 75.6%, which was more than 84 times that in THF. The single crystal structure of Cz1-TPAN revealed that the intermolecular interactions of C–H⋯π (2.242 Å, 2.889 Å), would lock the molecular conformation, thus yielding highly emissive behaviors in the crystals. Interestingly, Cz1-TPAN also exhibited mechanochromic behavior. The as-prepared crystals of Cz1-TPAN giving yellowish green fluorescence could be transformed into the powders emitting orange yellow light upon grinding, and the emission could be recovered when the ground powders were heated or fumed with DCM. This suggests that the reversible mechanochromism originates from the transformation between crystalline and amorphous states. It should be noted that Cz2-TPAN did not show mechanochromic properties. We deduced that compared with Cz1-TPAN, Cz2-TPAN had more a planar conjugated skeleton, which could give it compact intermolecular stacking and strong π–π interactions in the solid state, thus it exhibited no morphology change upon grinding.
Introduction
Mechanofluorochromic (MFC) materials have attracted a lot of attention over the past decade due to their promising applications in optical storage, pressure sensors, rewritable media, and security ink.1–3 These fluorescent organic molecules can show tunable and switchable solid-state luminescence under mechanical stress, and can be restored to their original state by annealing or fuming by solvent vapor.4 It was found that the changes in the solid emission were induced by the transformation of the molecular packing mode or molecular conformation instead of the chemical structure change under a pressure stimulus,5 so the mechanofluorochromism was usually reversible.Organic solid-state luminescent materials with electron donor (D) and electron acceptor (A) moieties are promising candidates because of their excellent optoelectronic properties and applications in organic light-emitting diodes (OLED),6 organic semiconductors,7 organic solid-state lasers8 and organic fluorescent sensors.9 Moreover, the electronic structures and thus optoelectronic properties of the D–π–A architectures can be facilely modulated through tuning the electron donor and acceptor strengths.10,11 However, the traditional aggregation-caused quenching (ACQ) effect in the solid state, owing to internal conversion, intersystem crossing, intramolecular charge transfer, intermolecular electron transfer, excimer or exciplex formation, or isomerisation, results in drastically negative effects on the device performance and sensitivity of the sensors.12 Since Tang's group reported that the restriction of intramolecular rotations was favourable for the generation of highly luminescent solid organic emitters, much effort has been expended to design new dyes with AIE (aggregation-induced emission).13 It is interesting that the mechanochromic luminogens are mostly AIE active. Till now, it has been found that the derivatives of tetraphenylethene,14 distyrylanthracene derivatives,15 β-diketone boron complexes16 and other architectures17 exhibit MFC properties because of their loose molecular packing in crystals which can be damaged easily under external stimuli. In our previous work, we have reported that phenothiazine and triphenylamine functionalized benzoxazole derivatives with ICT emission exhibited mechanochromism.18 At the same time our group has demonstrated that carbazole and tert-butyl carbazole functionalized β-iminoenolate boron complexes with strong ICT emission have different mechanofluorochromic properties.19 Zhang et al. reported two luminogens consisting of bisarylamine and triphenylacrylonitrile (TPAN) units exhibited typical AIE characteristics and obvious mechanochromism.20 Therefore, the fabrication of AIE active D–π–A molecules is a rational and promising choice. On the one hand, the carbazole unit was introduced due to the strong electron donating ability and strong luminance.21 On the other hand, TPAN derivatives usually showed AIE properties and better electron-withdrawing ability.10 With these in mind, we designed and synthesized new D–π–A type carbazole modified triphenylacrylonitrile derivatives (Cz1-TPAN and Cz2-TPAN, Scheme 1). It was found that Cz1-TPAN and Cz2-TPAN exhibited ICT emission and AIE characteristics with high solid-state efficiency (the fluorescence quantum yields of Cz1-TPAN and Cz2-TPAN were 75.6% and 33.5%, respectively). Moreover, Cz1-TPAN showed significant MFC property. We found that the as-prepared crystals of Cz1-TPAN exhibited yellowish green fluorescence and could be transformed into the ground powders emitting orange yellow light upon grinding. After fuming with DCM or heating, the crystalline structures were recovered, and they emitted bright yellowish green light. The XRD patterns of Cz1-TPAN in different solid states illustrated that the reversible mechanochromism resulted from the transformation between crystalline and amorphous states. The single crystal structure of Cz1-TPAN revealed that the intermolecular interactions of C–H⋯π (2.242 Å, 2.889 Å), which rigidify the molecular conformation, thus rendering Cz1-TPAN highly emissive in the crystalline states. So the obtained Cz1-TPAN might be used as sensors and memory chips on the basis of the solid fluorescence in response to external mechanical forces and organic solvents. It should be noted that Cz2-TPAN did not show mechanochromic property. The as-synthesized powders exhibiting very broad diffraction peak were the amorphous states. We deduced that compared with Cz1-TPAN, Cz2-TPAN had more planar conjugated skeleton, which could render it compact intermolecular stacking and strong π–π interactions in the solid states, thus exhibited no morphology change upon grinding.
Experimental section
Materials and measurements
1H NMR spectra (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Bruker AMX-400 NMR spectrometer in DMSO-d6 as the solvent at room temperature. Mass spectra were performed on Agilent 1100 MS series and AXIMA CFR MALDI/TOF (Matrix assisted laser desorption ionization/Time-of-flight) mass spectrometers. FT-IR spectra were measured with a Nicolet-360 FT-IR spectrometer by the incorporation of samples in KBr disks. C, H and N Elemental analyses were taken on a Perkin-Elmer 240C elemental analyzer. The UV-vis absorption spectra were determined on a Beijing purkinje TU-1810 Spectrophotometer. Fluorescence emission spectra were obtained using Shimadzu RF-5301 PC Spectrofluorophotometer. The fluorescence quantum yields of Cz1-TPAN and Cz2-TPAN in solutions were estimated by comparing to a standard (quinine sulfate in 0.1 N H2SO4 aqueous solution, ΦF = 0.54). The excitation wavelength was 365 nm. The absolute fluorescence quantum yields were measured on an Edinburgh FLS920 steady state spectrometer using an integrating sphere. Cyclic voltammetry (CV) was performed using CHI 604C electrochemical working station and measurements were carried out in DCM containing 0.1 M tetrabutylammonium hexafluorophosphate (Bu4NPF6) as a supporting electrolyte at room temperature. Platinum button was used as a working electrode, a platinum wire as a counter electrode and Ag/AgCl as the reference electrode. The scan rate was maintained at 50 mV s−1. The XRD patterns were obtained on an Empyrean X-ray diffraction instrument equipped with graphite-mono-chromatized CuKα radiation (λ = 1.5418 Å), by employing a scanning rate of 0.026° s−1 in the 2θ range from 10° to 40°, and the samples of the as-synthesized crystals, ground solids and fuming samples on glass slides were determined at room temperature. Single crystal was obtained by slow solvent evaporation from the solution in DCM at room temperature. Single crystal of Cz1-TPAN was selected for X-ray diffraction studies in a Rigaku RAXIS-RAPID diffractometer using graphite-monochromated MoKα radiation (λ = 0.71073 Å). The single crystal was kept at room temperature during the data collection. The structure was solved by direct methods and refined on F2 by full-matrix least-square using the SHELXTL-97 program. Differential scanning calorimetry (DSC) curves were determined using a NETZSCH STA499F3 QMS403D/Bruker V70 at a heating rate of 10 °C min−1. The frontier orbitals of Cz1-TPAN and Cz2-TPAN were obtained by density functional theory (DFT) calculations at the B3LYP/6-31G level with the Gaussian 09W program package. Tetrahydrofuran (THF) was distilled over sodium and benzophenone. DMF was distilled from phosphorous pentoxide, and other chemicals and reagents were used as received without further purification.Preparation of aggregates for AIE measurement
A stock solution of the samples in THF with a concentration of 2.0 × 10−4 M was prepared. An aliquot (1 mL) of the stock solution was transferred to a 10 mL volumetric flask. After an appropriate amount of THF was added, water was added dropwise under vigorous stirring to furnish the mixture with different water fractions. The concentration was maintained at 2.0 × 10−5 M. The emission measurement of the resultant mixtures was performed immediately.Preparation of the samples for mechanochromism study
The ground powders were prepared by grinding the as-synthesized crystals with a pestle in the mortar for 3 min. The fumed samples were obtained by fuming the ground powders with DCM for 30 s. The ground powders were heated at a certain temperature until the emission was totally recovered.Synthetic procedures and characterizations
Results and discussion
Synthesis
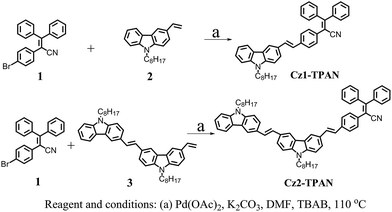
The synthetic routes for Cz1-TPAN and Cz2-TPAN are shown in Scheme 1. Firstly, 2-(4-bromophenyl)-3,3-diphenylacrylonitrile 1,22 9-octyl-3-vinyl-9H-carbazole 2,23 9-octyl-3-((E)-2-(9-octyl-9H-carbazol-6-yl)vinyl)-6-vinyl-9H-carbazole 3 (ref. 23) were prepared according to the reported methods. Then, the target molecular Cz1-TPAN was prepared via Heck reaction between compounds 1 and 2 catalyzed by Pd(OAc)2 at 110 °C for 10 h in a yield of 85%. Similarly, Cz2-TPAN was obtained by Heck reaction in a yield of 60%. All the intermediates and the final products were purified by column chromatography, and the new compounds were characterized by 1H-NMR, 13C-NMR, FT-IR and MALDI-TOF mass spectrometry. In the FT-IR spectra of compounds Cz1-TPAN and Cz2-TPAN, the vibration absorption bands appeared at ca. 960 cm−1, suggesting that C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond was in trans-form.24 Meanwhile, 1H NMR spectra of Cz1-TPAN and Cz2-TPAN also confirmed that C
C bond was in trans-form.24 Meanwhile, 1H NMR spectra of Cz1-TPAN and Cz2-TPAN also confirmed that C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups adopted the trans-conformation on account of the absence of the signal at ∼6.5 ppm assigned to the protons in cis-double bonds (CH
C groups adopted the trans-conformation on account of the absence of the signal at ∼6.5 ppm assigned to the protons in cis-double bonds (CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH).24,25 In addition, the single crystal structure of Cz1-TPAN was obtained. The obtained compounds Cz1-TPAN and Cz2-TPAN showed good solubility in common organic solvents, such as THF, CH2Cl2, CHCl3, DMF and DMSO.
CH).24,25 In addition, the single crystal structure of Cz1-TPAN was obtained. The obtained compounds Cz1-TPAN and Cz2-TPAN showed good solubility in common organic solvents, such as THF, CH2Cl2, CHCl3, DMF and DMSO.
Photophysical properties
The UV-vis absorption spectra of Cz1-TPAN and Cz2-TPAN in THF (2.0 × 10−5 M) are shown in Fig. 1. Two absorption bands at ca. 300 nm and 389 nm appeared for Cz1-TPAN, and the former band could be ascribed to the π–π* transitions, whereas the latter was assignable to the ICT transition.26 It was clear that Cz2-TPAN exhibited four absorption bands located at ca. 310 nm, 343 nm and 394 nm, as well as a shoulders band at ca. 330 nm. The absorption bands at 310 nm and 343 nm might be attributed to the π–π* transition, and the one at 394 nm corresponded to ICT transition. The red shift in the π–π* transitions absorption wavelength of Cz2-TPAN than Cz1-TPAN was attributed to the extension of π-conjugation, which could be confirmed by the calculated optimized molecular structure (Fig. S7†). As a result, the more planar conformation would lead to more conjugation of Cz2-TPAN with ICT features. Normally, photophysical properties of D–π–A conjugates are highly dependent on the solvent polarity. In order to confirm the occurrence of ICT transition, the solvent-dependent UV-vis absorption and fluorescence emission spectra of Cz1-TPAN and Cz2-TPAN are shown in Fig. S8 and S9.† It was clear that the slight red-shift of the absorption in the long wavelength region was detected by increasing the solvent polarity. In the case of Cz1-TPAN, the maximum absorption peak at 383 nm in hexane shifted to 390 nm in DMF. Cz2-TPAN showed similar absorption profiles in varying solvents, indicating their solvents polarity independent ground state electronic structures and small dipole moments associated with the ICT transitions. Additionally, we found that the emission bands of Cz1-TPAN and Cz2-TPAN red-shifted and broadened significantly in more polar solvents compared with those in non-polar solvents. For example, the maximum emission peak of Cz1-TPAN was located at 471 nm in hexane, and red-shifted to 563 nm in DMF. The above electronic spectral changes with solvent polarities suggested that the absorption at 389 nm for Cz1-TPAN and at 394 nm for Cz2-TPAN was due to ICT transition. Moreover, the ICT absorption band of Cz2-TPAN red-shifted to 394 nm compared with that of Cz1-TPAN (389 nm), indicating the strong electron donating ability of Cz2-TPAN.Theoretical calculation
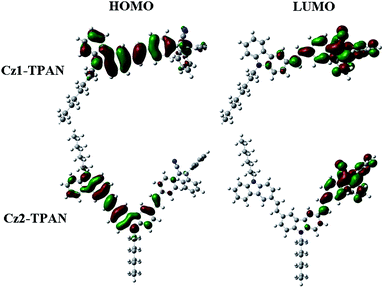
To further clarify the ICT transition, we carried out the density functional theory (DFT) calculations for Cz1-TPAN and Cz2-TPAN by Gaussian 09W program using DFT/B3LYP/6-31G method to reveal their electronic structures. The frontier orbital plots of the HOMO and LUMO are shown in Fig. 2, and the corresponding energy levels are listed in Table S1.† We could find that the HOMO levels for Cz1-TPAN and Cz2-TPAN were mainly located at the carbazole and vinyl units, while the LUMO levels were mainly distributed at the acceptor of the TPAN moiety. These results demonstrated that Cz1-TPAN and Cz2-TPAN were typical D–π–A molecules. As a result, the intramolecular charge transfer would occur in the D–π–A type carbazole-based triphenylacrylonitrile derivatives. Moreover, the calculated energy levels of HOMO and LUMO for Cz1-TPAN and Cz2-TPAN were close to those based on CV results (Table S1†).Electrochemical properties
The electrochemical behaviors of Cz1-TPAN and Cz2-TPAN were examined by cyclic voltammetry (CV) using a standard three-electrode cell and an electrochemical workstation (CHI 604C) under N2 atmosphere. As shown in Fig. S10,† it was clear that Cz1-TPAN and Cz2-TPAN exhibited two reversible oxidation processes with first half-wave oxidation potentials were located at +0.88 V and +0.68 V (vs. Fc/Fc+). On the basis of the first half-wave oxidation potentials, the highest occupied molecular orbital (HOMO) energy levels could be estimated as −5.29 eV for Cz1-TPAN and −5.09 eV for Cz2-TPAN. The HOMO energy level of Cz2-TPAN was higher than that of Cz1-TPAN due to the stronger electron donating ability. The lowest unoccupied molecular orbital (LUMO) energy level of these compounds could be estimated from the HOMO energy level and energy band gap (Eg) as the following equation: ELUMO = EHOMO + Eg, in which Eg was estimated from the onset of the absorption spectrum (Eg = 1240/λonset). It was found that the LUMO energy levels were −2.51 eV and −2.35 eV for Cz1-TPAN and Cz2-TPAN (Table S1†).AIE properties
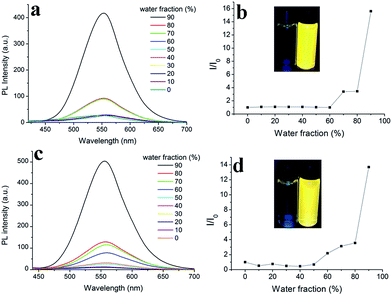
Triphenylacrylonitrile (TPAN) is a typical AIE compound.27 It is thus envisioned that Cz1-TPAN and Cz2-TPAN are also AIE-active. To verify this, fluorescence spectra of Cz1-TPAN and Cz2-TPAN in THF and THF–water mixtures were measured. Water is chosen because it is a typical non-solvents for the compounds: the luminogen molecules must aggregate in the THF–water mixtures with high water fraction (fw). As shown in Fig. 3, Cz1-TPAN in pure THF and THF–water mixtures (fw) ≤ 80% exhibited extremely weak PL signals, which was ascribed to the active intramolecular rotations of the genuinely dissolved luminogens in these mixtures. However, when fw was increased to 90%, the emission at ca. 553 nm was boosted swiftly and its intensity was ca. 15.6-fold higher than that in THF. It illustrated that Cz1-TPAN molecules tended to aggregate when a large amount of non-solvent of water (fw > 80%) was added in the mixture, in which the encapsulated Cz1-TPAN molecules were located in a non-polar environment and the ICT process was limited. Thus, the fluorescence was lightened.28 Similarly, the emission of Cz2-TPAN was also very weak in THF, and it intensified significantly in THF/water (fw > 80%). Around 13.7-fold of emission enhancement was observed for Cz2-TPAN when fw reached 90% in THF/water systems, and orange yellow light (centered at 556 nm) emitting was observed for the aggregates. We deem that in THF and low fw mixtures, these molecules are still dissolved in molecular form. The intramolecular rotations of the molecules are highly active, which effectively consumes the exciton energies, thus making the molecules almost non-emissive. In high fw mixtures, due to the reduction in the solvating power of the aqueous media, these molecules start to aggregate, which greatly impedes the intramolecular rotations of the molecules. It renders the excitons deactivated mainly through the radiative path, thus boosting the emission.20To quantitatively evaluate AIE, the fluorescence quantum efficiencies of the luminogens in solutions (ΦF,s) and as-prepared solid (ΦF,aps) states were determined. While ΦF,s values for Cz1-TPAN and Cz2-TPAN in THF were as low as 0.90% and 0.97%, ΦF,aps were boosted to 75.6% and 33.5%, respectively, further suggesting AIE behaviors. Moreover, we could get the corresponding AIE factors (αAIE = ΦF,aps/ΦF,s) of 84.0 and 34.5. It illustrated that Cz1-TPAN and Cz2-TPAN had high solid state efficiency for ICT emitters.
Mechanochromic property
We previously found that some phenothiazine modified triphenylacrylonitrile compounds exhibited MFC properties and the non-planar architecture of phenothiazine as well as the ICT feature of the D–π–A system were favorable for mechanofluorochromism.29 Herein, we investigated the mechanofluorochromic properties of these compounds and found that Cz1-TPAN gave different emitting behaviors upon the treatment of grinding and fuming. It was clear from the inset of Fig. 4 that Cz1-TPAN exhibited high contract MFC behavior. Obvious difference of the emitting colors was detected in the as-synthesized crystals and ground powders of Cz1-TPAN. To further reveal the MFC property of Cz1-TPAN, the fluorescence emission spectra in different solid states are shown in Fig. 4. It was clear that the as-synthesized crystals of Cz1-TPAN gave one emission band at ca. 517 nm, and emitting color was intense yellowish green. When the as-synthesized crystals were ground, Cz1-TPAN emitted orange yellow light centered at ca. 547 nm. Moreover, the emitting color could be recovered to yellowish green (ca. 513 nm) after the ground powders of Cz1-TPAN were fumed with DCM. If the fumed powders of Cz1-TPAN were reground, its fluorescence red-shifted to 551 nm again. It was found that the emitting color of Cz1-TPAN changed from orange yellow to yellowish green (ca. 520 nm) after heating. As a result, the emitting color of Cz1-TPAN could be transferred between yellowish green and orange yellow reversibly through grinding and heating/fuming treatment, suggesting the reversibility of the mechanochromic fluorescence (Fig. S11†). In addition, we found that Cz1-TPAN had a high fluorescence quantum yields (ΦF) in the as-synthesized crystals (75.6%) and in the ground powders (65.2%). It suggested that the synthesized Cz1-TPAN could be used as solid emitting materials.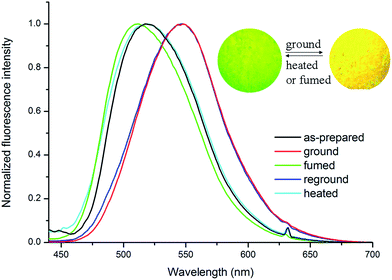 | ||
| Fig. 4 Fluorescence emission spectra of Cz1-TPAN excited at 400 nm; insets are photographs of Cz1-TPAN in different solid states irradiated at 365 nm. | ||
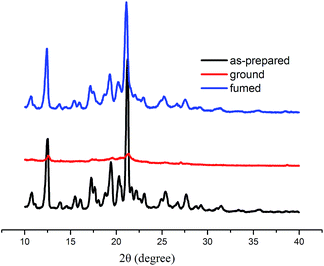
To gain an insight into the MFC behavior of Cz1-TPAN solids, powder wide-angle X-ray diffraction experiments were conduced on the as-prepared, ground and fumed solids. As shown in Fig. 5, the as-prepared crystals of Cz1-TPAN exhibited many intense and sharp diffraction peaks, which were indicative of their regular crystalline structure, after grinding, the ground solids showed rather weak signals, indicating disordered molecular packing.30 However, when heated or fumed with solvent, sharp diffractions emerged again, implying the recovery of an ordered crystalline lattice. As a result, we deduced that the mechanochromism of Cz1-TPAN was due to the transition between the ordered crystalline and the disordered amorphous states. Furthermore, the results clearly indicated that mechanochromism was highly associated with the molecular arrangement that greatly influenced properties.31 It should be noted that Cz2-TPAN did not show mechanochromic property. As shown in Fig. S12,† the as-synthesized powders exhibiting very broad diffraction peak were the amorphous states.32 We deduced that compared with Cz1-TPAN, Cz2-TPAN had more planar conjugation skeleton, which could render it compact intermolecular stacking and strong π–π interactions in the solid states, thus exhibited no morphology change upon grinding. This work demonstrates once again that conjugated organic molecules exhibiting aggregation-induced emission (AIE) are characterized by the strongly twisted conjugated skeleton, which could provide them loose intermolecular stacking and weak π–π interactions in the solid states. It provided a strategy for designing new dyes with stable or reversible mechanochromic materials.
To further reveal the effect of heating on the mechanochromic property, the differential scanning calorimetry (DSC) curves are shown in Fig. 6. The as-synthesized crystals of Cz1-TPAN gave only one strong endothermic peak at 151 °C, corresponding to its melting point, while one weak exothermic transition peak at 81 °C appeared in the ground powders of Cz1-TPAN, which could be ascribed to the cold-crystallization (crystallizing from glass state) of the ground sample upon heat annealing.33 It illustrated that the amorphous state was a metastable state. It should be noted that the exothermic peaks for the amorphous powders of Cz1-TPAN appeared at high temperature (81 °C), which resulted in its stable MFC behavior at room temperature.
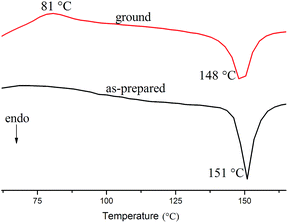 | ||
| Fig. 6 DSC curves of Cz1-TPAN in the as-prepared crystals (black) and ground powders (red) under nitrogen atmosphere at a heating rate of 10 °C min−1. | ||
The single crystal structure of Cz1-TPAN was obtained to further understand its AIE and mechanochromic behavior. As shown in Fig. 7 and S13,† it adopted highly nonplanar conformation, which favored active intramolecular rotations in solutions, thus effectively consuming exciton energies and subsequently making the molecules non-luminescent in solutions. Upon aggregation, these intramolecular rotations were highly impeded. Meanwhile, no π–π stacking between aromatic rings were formed owing to its twisted conformation, thus giving greatly boosted emissions. Moreover, in the single crystal of Cz1-TPAN, the intermolecular interactions of C–H⋯π (2.242 Å, 2.889 Å) were observed (Fig. 7b). These kinds of intermolecular interactions would rigidify the molecular conformation and prohibit the intramolecular rotation, leading to highly emissive behaviors in the crystals. Upon grinding, some of the interactions were destroyed, and the molecules would be forced to adopt less twisted conformation, thus yielding a red-shift and relative weak fluorescence.34
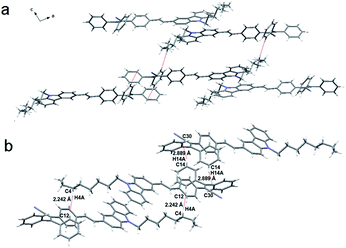 | ||
| Fig. 7 (a) Molecular packing of Cz1-TPAN in single crystal and (b) the intermolecular interactions in single crystal of Cz1-TPAN. | ||
Conclusions
In summary, new D–π–A luminogens based on carbazole modified triphenylacrylonitrile derivatives Cz1-TPAN and Cz2-TPAN have been designed and synthesized by the Pd-catalyzed Heck cross-coupling reaction. It was found that Cz1-TPAN and Cz2-TPAN exhibited ICT emission. It was interesting that although their emissions in solutions were very weak, we observed strong emissions in aggregate state and solid state. These compounds exhibited obvious AIE behaviors. For example, the ΦF of Cz1-TPAN in as-synthesized crystals reached 75.6%, which was more than 84 times of that in THF. Moreover, Cz1-TPAN displayed significant MFC property, the synthesized crystals of Cz1-TPAN could emit intense yellowish-green light under UV irradiation. After grinding, the emitting colors of Cz1-TPAN would change into orange yellow light. The mechanochromism was reversible upon the treatment of grinding and heating/fuming with DCM on account of the transition between the crystalline and amorphous states. The single crystal X-ray structure of Cz1-TPAN revealed that the intermolecular interactions of C–H⋯π (2.242 Å, 2.889 Å) would lead to the increased rigidity of the molecular skeleton, which would restrict the intramolecular rotation, yielding intense emission in crystals. It should be noted that Cz2-TPAN did not show mechanochromic property. The as-synthesized powders exhibiting very broad diffraction peak were the amorphous states. We deduced that compared with Cz1-TPAN, Cz2-TPAN had more planar conjugated skeleton, which could render it compact intermolecular stacking and strong π–π interactions in the solid states, thus exhibited no morphology change upon grinding. It provided a strategy for designing new dyes with stable or reversible mechanochromism emitters, which might be used as sensors and memory chips on the basis of the solid fluorescence in response to external mechanical forces and organic solvents.Acknowledgements
This work is financially supported by Scientific Research Fund of Liaoning Provincial Education Department of China (No. L2015528) and Liaoning Provincial Nature Science Foundation of China (No. 2015020755).Notes and references
- (a) Z. Chi, X. Zhang, B. Xu, X. Zhou, C. Ma, Y. Zhang, S. Liu and J. Xu, Chem. Soc. Rev., 2012, 41, 3878 RSC; (b) Q. K. Qi, J. Y. Qian, J. B. Zhang, L. J. Wang, B. Xu, B. Zou and W. J. Tian, Adv. Funct. Mater., 2015, 25, 4005 CrossRef CAS.
- (a) X. Q. Zhang, Z. Y. Ma, Y. Yang, X. Y. Zhang, X. R. Jia and Y. Wei, J. Mater. Chem. C, 2014, 2, 8932 RSC; (b) X. Zhang, Z. Chi, Y. Zhang, S. Liu and J. Xu, J. Mater. Chem. C, 2013, 1, 3376 RSC; (c) X. Q. Zhang, X. Y. Zhang, L. Tao, Z. G. Chi, J. R. Xu and Y. Wei, J. Mater. Chem. B, 2014, 2, 4398 RSC.
- (a) F. Ciardelli, G. Ruggeri and A. Pucci, Chem. Soc. Rev., 2013, 42, 857 RSC; (b) Z. Q. Zhang, Z. Wu, J. B. Sun, B. Q. Yao, G. H. Zhang, P. C. Xue and R. Lu, J. Mater. Chem. C, 2015, 3, 4921 RSC.
- (a) Z. Chem, Z. Li, F. Hu, G. A. Yu, J. Yin and S. H. Liu, Dyes Pigm., 2016, 125, 169 CrossRef; (b) P. Baranyai, G. Marsi, C. Jobbágy, A. Domján, L. Oláh and A. Deák, Dalton Trans., 2015, 44, 13455 RSC.
- (a) Z. Ma, M. Teng, Z. Wang, S. Yang and X. Jia, Angew. Chem., Int. Ed., 2013, 52, 12268 CrossRef CAS PubMed; (b) J. R. Kumpfer, S. D. Taylor, W. B. Connick and S. J. Rowan, J. Mater. Chem., 2012, 22, 14196 RSC; (c) R. Yoshii, A. Nagai, K. Tanaka and Y. Chujo, Chem.–Eur. J., 2013, 19, 4506 CrossRef CAS PubMed; (d) Z. K. He, L. Q. Zhang, J. Mei, T. Zhang, J. W. Y. Lam, Z. G. Shuai, Y. Q. Dong and B. Z. Tang, Chem. Mater., 2015, 27, 6601 CrossRef CAS; (e) J. X. Wu, J. Tang, H. L. Wang, Q. K. Qi, X. F. Fang, Y. F. Liu, S. P. Xu, X. A. Zhang, H. Y. Zhang and W. Q. Xu, J. Phys. Chem. A, 2015, 119, 9218 CrossRef CAS PubMed.
- (a) A. C. Grimsdale, K. L. Chan, R. E. Martin, P. G. Jokisz and A. B. Holmes, Chem. Rev., 2009, 109, 897 CrossRef CAS PubMed; (b) P. L. Burn, S. C. Lo and I. D. W. Samuel, Adv. Mater., 2007, 19, 1675 CrossRef CAS.
- A. R. Murphy and J. M. J. Fréchet, Chem. Rev., 2007, 107, 1066 CrossRef CAS PubMed.
- M. D. McGehee and A. J. Heeger, Adv. Mater., 2000, 12, 1655 CrossRef CAS.
- T. J. Dale and J. Rebek, J. Am. Chem. Soc., 2006, 128, 4500 CrossRef CAS PubMed.
- Y. Y. Gong, Y. Q. Tan, J. Liu, P. Lu, C. F. Feng, W. Z. Yuan, Y. W. Lu, J. Z. Sun, G. F. He and Y. M. Zhang, Chem. Commun., 2013, 49, 4009 RSC.
- M. Pawlicki, H. A. Collins, R. G. Denning and H. L. Anderson, Angew. Chem., Int. Ed., 2009, 48, 3244 CrossRef CAS PubMed.
- (a) P. C. Xue, J. B. Sun, P. Chen, P. Gong, B. Q. Yao, Z. Q. Zhang, C. Qian and R. Lu, J. Mater. Chem. C, 2015, 3, 4086 RSC; (b) J. B. Birks, Photophysics of Aromatic Molecules, Wiley, London, 1970 Search PubMed; (c) A. Qin and B. Z. Tang, Aggregation-induced emission: fundamentals, Wiley, United Kingdom, 2013 Search PubMed.
- (a) Y. N. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361 RSC; (b) R. R. Hu, N. L. C. Leung and B. Z. Tang, Chem. Soc. Rev., 2014, 43, 4494 RSC.
- (a) X. Q. Zhang, Z. G. Chi, B. J. Xu, L. Jiang, X. Zhou, Y. Zhang, S. W. Liu and J. R. Xu, Chem. Commun., 2012, 48, 10895 RSC; (b) H. Y. Li, X. Q. Zhang, Z. G. Chi, B. J. Xu, W. Zhou, S. W. Liu, Y. Zhang and J. R. Xu, Org. Lett., 2011, 13, 556 CrossRef CAS PubMed; (c) Y. J. Dong, J. B. Zhang, X. Tan, L. J. Wang, J. L. Chen, B. Li, L. Ye, B. Xu, B. Zou and W. J. Tian, J. Mater. Chem. C, 2013, 1, 7554 RSC; (d) Y. J. Dong, B. Xu, J. B. Zhang, X. Tan, L. J. Wang, J. L. Chen, H. G. Lv, S. P. Wen, B. Li, L. Ye, B. Zou and W. J. Tian, Angew. Chem., Int. Ed., 2012, 51, 10781 Search PubMed.
- (a) C. P. Ma, B. J. Xu, G. Y. Xie, J. J. He, X. Zhou, B. Y. Peng, L. Jiang, B. Xu, W. J. Tian, Z. G. Chi, S. W. Liu, Y. Zhang and J. R. Xu, Chem. Commun., 2014, 50, 7374 RSC; (b) J. X. Wu, J. Tang, H. L. Wang, Q. K. Qi, X. F. Fang, Y. F. Liu, S. P. Xu, X. A. Zhang, H. Y. Zhang and W. Q. Xu, J. Phys. Chem. A, 2015, 119, 9218 CrossRef CAS PubMed; (c) Y. Wang, I. Zhang, B. H. Yu, X. F. Fang, X. Su, Y. M. Zhang, T. Zhang, B. Yang, M. J. Li and X. A. Zhang, J. Mater. Chem. C, 2015, 3, 12328 RSC.
- (a) P. Galer, R. C. Korošec, M. Vidmar and B. Šket, J. Am. Chem. Soc., 2014, 136, 7383 CrossRef CAS PubMed; (b) T. Liu, A. D. Chien, J. Lu, G. Zhang and C. L. Fraser, J. Mater. Chem., 2011, 21, 8401 RSC; (c) G. Zhang, J. Lu, M. Sabat and C. L. Fraser, J. Am. Chem. Soc., 2010, 132, 2160 CrossRef CAS PubMed.
- (a) S. J. Yoon and S. Y. Park, J. Mater. Chem., 2011, 21, 8338 RSC; (b) J. W. Sun, X. J. Lv, P. J. Wang, Y. J. Zhang, Y. Y. Dai, Q. C. Wu, M. Ouyang and C. Zhang, J. Mater. Chem. C, 2014, 2, 5365 RSC; (c) Z. Chen, J. Zhang, M. Song, J. Yin, G. A. Yu and S. H. Liu, Chem. Commun., 2015, 51, 326 RSC; (d) P. Gong, H. Yang, J. B. Sun, Z. Q. Zhang, J. B. Sun, P. C. Xue and R. Lu, J. Mater. Chem. C, 2015, 3, 10302 RSC; (e) Y. J. Zhang, Q. B. Song, K. Wang, W. G. Mao, F. Cao, J. W. Sun, L. L. Zhan, Y. K. Lv, Y. G. Ma, B. Zou and C. Zhang, J. Mater. Chem. C, 2015, 3, 3049 RSC.
- (a) P. C. Xue, P. Chen, J. H. Jia, Q. X. Xu, J. B. Sun, B. Q. Yao, Z. Q. Zhang and R. Lu, Chem. Commun., 2014, 50, 2569 RSC; (b) P. C. Xue, B. Q. Yao, J. B. Sun, Q. X. Xu, P. Chen, Z. Q. Zhang and R. Lu, J. Mater. Chem. C, 2014, 2, 3942 RSC.
- Z. Q. Zhang, P. C. Xue, P. Gong, G. H. Zhang, J. Peng and R. Lu, J. Mater. Chem. C, 2014, 2, 9543 RSC.
- Y. Y. Gong, J. Liu, Y. R. Zhang, G. F. He, Y. Lu, W. B. Fan, W. Z. Yuan, J. Z. Sun and Y. M. Zhang, J. Mater. Chem. C, 2014, 2, 7552 RSC.
- (a) T. H. Xu, R. Lu, X. L. Liu, P. Chen, X. P. Qiu and Y. Y. Zhao, J. Org. Chem., 2008, 73, 1809 CrossRef CAS PubMed; (b) X. L. Liu, R. Lu, T. H. Xu, D. F. Xu, Y. Zhan, P. Chen, X. P. Qiu and Y. Y. Zhao, Eur. J. Org. Chem., 2009, 53 Search PubMed; (c) X. L. Liu, X. F. Zhang, R. Lu, P. C. Xue, D. F. Xu and H. P. Zhou, J. Mater. Chem., 2011, 21, 8756 RSC.
- W. Z. Yang, Y. Gong, S. Chen, X. Y. Shen, J. W. Y. Lam, P. Lu, Y. Lu, Z. Wang, R. Hu, N. Xie, H. Kwok, Y. Zhang, J. Z. Sun and B. Z. Tang, Chem. Mater., 2012, 24, 1518 CrossRef.
- (a) Y. Zhan, K. Y. Cao, C. G. Wang, J. H. Jia, P. C. Xue, X. L. Liu, X. M. Duan and R. Lu, Org. Biomol. Chem., 2012, 10, 8701 RSC; (b) Y. Zhan, J. Peng, K. Q. Ye, P. C. Xue and R. Lu, Org. Biomol. Chem., 2013, 11, 6814 RSC; (c) J. Peng, K. Q. Ye, G. H. Zhang, Y. Zhan, J. H. Jia, P. C. Xue and R. Lu, Synth. Met., 2014, 193, 94 CrossRef CAS.
- (a) X. P. Qiu, R. Lu, H. P. Zhou, X. F. Zhang, T. H. Xu, X. L. Liu and Y. Y. Zhao, Tetrahedron Lett., 2007, 48, 7582 CrossRef CAS; (b) X. Zhao, K. Y. Cao, H. P. Zhou and R. Lu, Opt. Mater., 2014, 5, 950 CrossRef; (c) H. P. Zhou, P. C. Xue, Y. Zhang, X. Zhao, J. H. Jia, X. F. Zhang, X. L. Liu and R. Lu, Tetrahedron, 2011, 44, 8477 CrossRef.
- (a) B. S. Li, J. Li, Y. Q. Fu and Z. S. Bo, J. Am. Chem. Soc., 2004, 126, 3430 CrossRef CAS PubMed; (b) B. Li, X. Xu, M. Sun, Y. Fu, G. Yu, Y. Liu and Z. S. Bo, Macromolecules, 2006, 39, 456 CrossRef CAS.
- (a) P. C. Xue, Q. X. Xu, P. Gong, C. Qian, Z. Q. Zhang, J. H. Jia, X. Zhao, R. Lu, A. M. Ren and T. R. Zhang, RSC Adv., 2013, 3, 26403 RSC; (b) X. L. Liu, D. F. Xu, R. Lu, B. Li, C. Qian, P. C. Xue, X. F. Zhang and H. P. Zhou, Chem.–Eur. J., 2011, 17, 1660 CrossRef CAS PubMed.
- Y. Y. Gong, Y. R. Zhang, W. Z. Yuan, J. Z. Sun and Y. M. Zhang, J. Phys. Chem. C, 2014, 118, 10998 CAS.
- X. Y. Shen, Y. J. Wang, E. Zhao, W. Z. Yuan, Y. Liu, P. Lu, A. Qin, Y. Ma, J. Z. Sun and B. Z. Tang, J. Phys. Chem. C, 2013, 117, 7334 CAS.
- G. H. Zhang, J. B. Sun, P. C. Xue, Z. Q. Zhang, P. Gong, J. Peng and R. Lu, J. Mater. Chem. C, 2015, 3, 2925 RSC.
- (a) B. Zhang, C. Hsu, S. Yu, S. Yang and E. Chen, Chem. Commun., 2013, 49, 8872 RSC; (b) T. Wen, D. Zhang, J. Liu, R. Lin and J. Zhang, Chem. Commun., 2013, 49, 5660 RSC.
- H. Zhang, Z. Zhang, K. Ye, J. Zhang and Y. Wang, Adv. Mater., 2006, 18, 2369 CrossRef CAS.
- (a) Y. J. Zhang, G. L. Zhuang, M. Ouyang, B. Hu, Q. B. Song, J. W. Sun, C. Zhang, C. Gu, Y. X. Xu and Y. G. Ma, Dyes Pigm., 2013, 98, 486 CrossRef CAS; (b) A. Rananaware, D. D. La and S. V. Bhosale, RSC Adv., 2015, 5, 56270 RSC.
- D. T. Zhang, Y. Y. Gao, J. Dong, Q. K. Sun, W. Liu, S. F. Xue and W. J. Yang, Dyes Pigm., 2015, 113, 307 CrossRef CAS.
- W. Z. Yuan, Y. Tan, Y. Gong, P. Lu, J. W. Y. Lam, X. Y. Shen, C. Feng, H. H. Y. Sung, Y. Lu, I. D. Williams, J. Z. Sun, Y. Zhang and B. Z. Tang, Adv. Mater., 2013, 25, 2837 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H-NMR, 13C-NMR and MALDI/TOF MS; normalized UV-vis absorption and fluorescence emission spectra of Cz1-TPAN and Cz2-TPAN in different solvents; cyclic voltammetry diagrams; XRD patterns of Cz2-TPAN in as-synthesized solid states; reversible switch of the emission upon grinding-fuming or heating; the preferred conformation of Cz1-TPAN. CCDC 1451514. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra03310e |
| This journal is © The Royal Society of Chemistry 2016 |