Effect of ammonium groups on the properties of anion conductive membranes based on partially fluorinated aromatic polymers†
Abstract
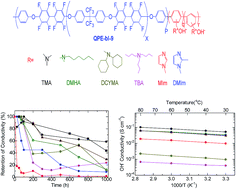
A series of anion exchange membranes (QPE-bl-9) based on a partially fluorinated hydrophobic segment and oligophenylene as scaffolds for ammonium cations were synthesized to evaluate the effect of the various ammonium groups derived from trimethyl amine (TMA), dimethyl hexyl amine (DMHA), methyl imidazole (MIm), dimethyl imidazole (DMIm), tributyl amine (TBA), and dicyclohexyl methyl amine (DCHMA) on the membrane properties. QPE-bl-9 membranes were well characterized by 1H NMR spectroscopy, in which all the peaks were assigned to the supposed structure. The TEM images of QPE-bl-9–TMA, –MIm, –DMIm and –DMHA membranes showed small hydrophilic/hydrophobic phase separated morphology (hydrophilic domains 1–3 nm). QPE-bl-9–TMA (1.6 mequiv. g−1) exhibited the highest hydroxide ion conductivity (101 mS cm−1 at 80 °C) among the tested membranes, followed by QPE-bl-9–DMHA (62 mS cm−1) and QPE-bl-9–DMIm (62 mS cm−1). The alkaline stability of the membranes was tested in 1 M KOH at 60 °C for 1000 h. QPE-bl-9–TMA exhibited the highest retention of the conductivity (58%), which was higher than that of the Tokuyama A201 anion exchange membrane (29%). The post stability test IR analyses suggested that the major degradation mechanism of the QPE-bl-9 membranes in alkaline solution involved the decomposition of the ammonium groups. The QPE-bl-9 membranes retained their mechanical stability after the stability test, as proved by DMA analyses.


 Please wait while we load your content...
Please wait while we load your content...