Enhancing the biological properties of carbon nanofibers by controlling the crystallization of incorporated bioactive glass via silicon content
Abstract
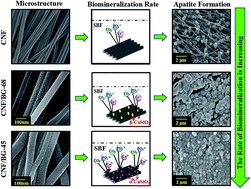
Bioactive glass (BG)-containing carbon nanofibers (CNFs) were prepared by combining the processes of sol–gel, polyacrylonitrile (PAN) electrospinning and heat treatment. Two types of BG, i.e. 45S and 68S, were incorporated. The crystalline structure evolution of the BG component during the formation of the CNFs was characterized by XRD, SEM, and TEM observations in relation to silicon content. Then the apatite-forming ability of the hybridized CNF/BG in simulated body fluid was evaluated in relation to the crystalline structure of the BG component. Interactions between functional groups in PAN and BG sol–gel precursors were identified in the steps of electrospinning and heat treatment. As a result, the 45S-type BG containing less silicon formed α-CaSiO3, while the 68S-type BG containing more silicon transformed to β-CaSiO3 in the final hybridized CNF/BG upon carbonization. This difference led to different dissolution rates and osteocompatibility activities of the BG component from the hybrids, which regulated their capacities in inducing apatite deposition, proliferation and osteogenic differentiation of bone mesenchymal stromal cells.

 Please wait while we load your content...
Please wait while we load your content...