The green reduction of graphene oxide
Abstract
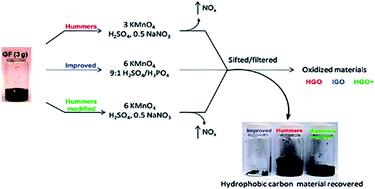
Graphene is an ultra-thin material, which has received broad interest in many areas of science and technology because of its unique physical, chemical, mechanical and thermal properties. Synthesis of high quality graphene in an inexpensive and eco-friendly manner is a big challenge. Among various methods, chemical synthesis is considered the best because it is easy, scalable, facile, and inexpensive. Different kinds of chemical reducers have been used to produce graphene sheets. However, some chemicals are toxic, corrosive, and hazardous. For this reason, researchers have been using different environmentally friendly substances (termed green reducers) to produce functional graphene sheets. This paper presents an overview and discussion of the green reduction of graphene oxide (GO) to graphene. It also reviews the characterization of GO and its oxide reduction through the analysis of different spectroscopic and microscopic techniques such as Raman spectroscopy, Fourier transform infrared spectroscopy, X-ray photoelectron spectroscopy, X-ray diffraction, transmission electron microscopy, scanning electron microscopy, and atomic force microscopy.

 Please wait while we load your content...
Please wait while we load your content...