Eu2+ → Tb3+ → Eu3+ energy transfer in Ca6La2Na2(PO4)6F2:Eu, Tb phosphors
Abstract
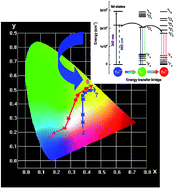
Novel phosphors Ca6La2Na2(PO4)6F2:0.10Eu2+/Eu3+, xTb3+ (x = 0, 0.05, 0.10, 0.20, 0.30, 0.40, 0.50) were prepared by a solid state reaction in a CO-reducing atmosphere. The fluorescence spectra of samples Ca6La2Na2(PO4)6F2:xEu2+ reveal that still a small number of Eu3+ ions are detected in the host. The Eu2+ → Tb3+ → Eu3+ energy transfer process in Ca6La2Na2(PO4)6F2:0.10Eu2+/Eu3+, xTb3+ phosphors was discussed in detail through the excitation, emission spectra and the fluorescence decay. As a result of Eu2+ sensitization, the relative emission intensity of Ca6La2Na2(PO4)6F2:0.10Eu2+/Eu3+, xTb3+ is enhanced under near-ultraviolet light excitation. Furthermore, tunable emission with a large color gamut can be obtained by changing the Tb3+ doping concentration. These results indicate that the Ca6La2Na2(PO4)6F2:Eu2+/Eu3+, Tb3+ phosphors will have potential use in n-UV chip pumped white LED devices.

 Please wait while we load your content...
Please wait while we load your content...