Preparation and application of novel biodegradable polyurethane copolymer
Abstract
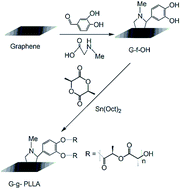
Polyurethanes and polylactides are widely used due to their excellent mechanical properties, biocompatibility, and flexible construction options and their degradable, hydrolyzable and biocompatible characteristics, respectively. Herein, a novel polyurethane copolymer, poly(L-lactide)-b-(4,4′-diphenylmethane diisocyanate)-functionalized graphene (PLLA-b-PU-f-G), was prepared by the ring-opening polymerization of LLA using phenol-functionalized graphene and tin octoate as the initiator and catalyst, respectively, followed by the condensation polymerization of OH-terminated poly(L-lactide)-functionalized graphene (G-g-PLLA) and 4,4′-diphenylmethane diisocyanate (MDI). The compositional analysis and structural characterization of the resulting materials were done by FTIR, 1H NMR, TG, and DSC. The FTIR and 1H NMR results clearly showed that graphene can be covalently functionalized with polyurethane and polylactides by the proposed approach. DSC analysis indicated that with increasing graphene content, the Tg value of G-g-PLLA tends to increase mildly. A simulative ocean hanging plate experiment indicated that the copolymer material has a better antifouling performance than polyurethane. A static hydrolysis experiment showed that the incorporation of graphene increased the hydrolysis ability of polyurethane, indicating that the functionalized polyurethane can be expected to serve as a marine antifouling coating by conferring a smooth surface.

 Please wait while we load your content...
Please wait while we load your content...