A colorimetric and fluorometric investigation of Cu(ii) ion in aqueous medium with a fluorescein-based chemosensor†
Abstract
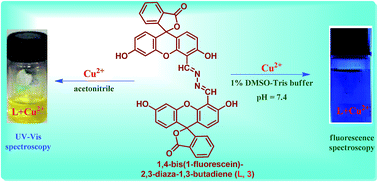
As a selective colorimetric and fluorometric chemosensor, a novel fluorescein-based Schiff base (FNSB), 1,4-bis(1-fluorescein)-2,3-diaza-1,3-butadiene (L, 3) exhibits efficient binding for Cu2+ ion in water and allows naked-eye detection as a result of the formation of a 1 : 1 copper–ligand complex. The complexations of Cu2+ ion due to molecular interaction with 3 resulted in a rapid change in color from colorless to deep yellow with a characteristic change in the UV-vis absorption to a longer wavelength by 130 nm in acetonitrile and significant quenching of the fluorescence intensity (∼2.5 fold for Cu2+ ion) with maxima at 519 nm in 1% DMSO–Tris buffer solution at physiological pH (7.4). The detection limit of Cu2+ ion in acetonitrile is found to be 20 μM by UV spectroscopy, whereas in aqueous solution, the limit is reduced to 1.25 μM by fluorescence spectroscopy.

 Please wait while we load your content...
Please wait while we load your content...