Is it possible to substitute hexane with green solvents for extraction of carotenoids? A theoretical versus experimental solubility study
Abstract
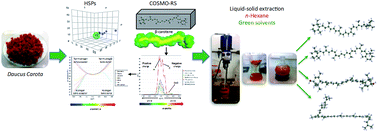
The present study was designed to evaluate five green solvents, i.e. 2-methyltetrahydrofuran (2-MeTHF), dimethyl carbonate (DMC), cyclopentyl methyl ether (CPME), isopropyl alcohol (IPA) and ethyl acetate, for the substitution of n-hexane in the extraction of carotenoids from carrots. Initially, solvent selection was made through the theoretical physicochemical solvent properties and solubility results obtained using two simulation programs, Hansen Solubility Parameters (HSPs) and Conductor-like Screening Model for Realistic Solvation (COSMO-RS) which use a statistical thermodynamics approach based on the result of quantum chemical calculation, for comprehension of the dissolving mechanism. On the basis of the HSPs analysis, non-polar or slightly polar solvents were the most suitable solvents for extraction of carotenoids. COSMO-RS analysis showed a higher probability of solubility for all the carotenoids from carrot in CPME, 2-MeTHF and ethyl acetate compared with n-hexane. The experimental results using a conventional solid–liquid extraction by maceration showed that the best green solvents were CPME, 2-MeTHF and ethyl acetate in accordance with the predictive results from COSMO-RS. The highest carotenoid content (78.4 mg 100 g−1 DM) was observed in CPME where 66% was represented by β-carotene and 34% was α-carotene. These results support the potential of CPME and 2-MeTHF as alternative green solvents for extraction of carotenoids.

 Please wait while we load your content...
Please wait while we load your content...