Binder-free lithium ion battery electrodes made of silicon and pyrolized lignin†
Tao Chen*,
Qinglin Zhang,
Jiagang Xu,
Jie Pan and
Yang-Tse Cheng
Department of Chemical and Materials Engineering, University of Kentucky, KY, USA 40506-0046. E-mail: tao.chen@uky.edu
First published on 16th March 2016
Abstract
The synthesis, characterization, and performance of a binder-free negative electrode for a lithium-ion battery, consisting of renewable biopolymer lignin and silicon nanoparticles, are reported. By mixing, coating, and subsequent pyrolization, we fabricated uniformly interconnected core–shell composite films of Si/C directly on the current collector, allowing for the assembly of coin-cells without the need of binder and conductive carbon. An excellent electrochemical performance was observed with a high specific capacity of 1557 mA h g−1 and a stable rate performance from 0.18 A g−1 to 1.44 A g−1. Moreover, the Si–pLig electrode can be reversibly cycled at 0.54 A g−1 with 89.3% capacity retention over 100 cycles. We also unveil a beneficial effect of 0.5% polyethylene oxide (PEO) on the morphology and electrochemical behavior of the Si/C composite electrodes.
The search for high energy density, low-cost, and environmentally friendly lithium ion batteries continues due to their numerous applications in, for example, electronic devices, electric vehicles, and implantable devices.1 Silicon is one of the most promising and challenging negative electrode active materials with a theoretical capacity of 4200 mA h g−1, more than ten times higher than that of graphite.2 Aside from its amazing theoretical capacity, silicon also boasts low lithium alloying/dealloying potential (about 370 mV vs. Li/Li+) and natural abundance. Unfortunately, a large capacity for silicon means a large volumetric change (300%) during charge/discharge which quickly results in cracking of the material and a rapid decline in capacity.3
A number of strategies have been deployed to accommodate the volume expansion and increase capacity retention of silicon anodes, including dispersion of silicon in inactive/active composites,4–7 development of novel binders,8–10 and growth of silicon thin films.11–13 Silicon/carbon composites are especially attractive because: (1) carbon is highly conductive, allowing for fast electron transport; (2) carbon is light and ductile, able to accommodate volume change; (3) carbon is known to form stable solid electrolyte interphases (SEIs) in aprotic organic solvents, which reduce capacity loss due to irreversible reactions.14 In addition, various silicon nanostructures have been proposed ranging from nanospheres, nanowires, nanotubes, to core–shells, greatly improving the performance and durability of silicon-based negative electrodes.15–23
Although improved performance over bulk and micro-sized silicon is achieved, the preparation of complicated nanostructures and formulations generally leads to increased cost which is undesirable since cost is one of the major factors limiting broad applications of batteries. Furthermore, in the conventional slurry coating process, costly polymeric binders and often toxic, volatile organic solvents were required, adding to the overall cost of batteries, hence the development of green and cost effective silicon anode processing techniques is essential.
Lignin is a renewable and abundant branched aromatic biopolymer which constitutes up to 30% of the organic carbon available. As a by-product of paper and pulp industry, lignin is produced in excess of 50 million tons annually.24 Lignin has been actively explored for generating high value-added products, notably carbon fibers.25–29 Its utilization allows the replacement of expensive polyacrylonitrile (PAN) or non-renewable petroleum-based pitch precursors.30 More recently, lignin has also been investigated as an energy storage material and lithium ion battery components. Milczarek and Inganas showed that lignosulfonate could be conjugated with conductive polymer to store charge via reversible quinone/hydroquinone chemistry.31 Using the melt-spinning technique, Tenhaeff et al. fabricated fused carbon fiber mats from lignin which could directly be used as lithium ion anode with comparable performance to traditional slurry coated graphite anode.32 Carbon fiber mats from lignin precursors were also prepared using electrospinning as free-standing electrodes for sodium ion batteries.33
In this report, we demonstrate a binder-free negative electrode for lithium ion battery consisting of renewable biopolymer lignin and nano-sized silicon nanoparticles. By mixing, coating, and subsequent pyrolization, we synthesized 3-dimensional, interconnected composite films of Si/C directly on the copper current collector, allowing for the assembly of coin-cells without the need of polymer binder and conductive carbon. We found that carbon from pyrolized lignin can provide conductive pathways for electrons and protect silicon nanoparticles from extensive SEI formation by forming a core–shell foam-like 3D network, while bonding the composite film securely onto the Cu current collector. As a result, excellent electrochemical performance was observed with a high specific capacity of 1557 mA h g−1, 89.3% capacity retention over 100 cycles, and good rate performance. We also unveil a beneficial effect of 0.5% polyethylene oxide (PEO) on the morphology and electrochemical behavior of Si/C composite electrodes.
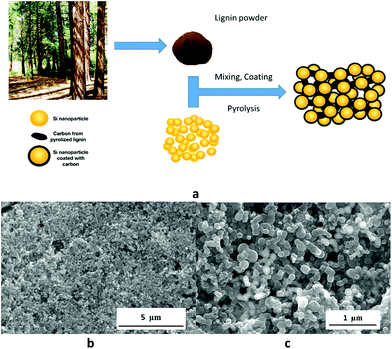
The concept of Si nanoparticle/pyrolized lignin composite (Si–pLig) is shown in Fig. 1a. A by-product of paper and pulp industry, lignin commonly from tree wood is commercially available as fine powder. Prior to pyrolization, Si nanoparticles are uniformly dispersed and coated with the heavily cross-linked lignin while lignin also formed a 3D matrix surrounding the Si nanoparticles. Upon pyrolization in an inert gas atmosphere, lignin is carbonized leaving behind a porous carbon network with graphitic and non-graphitic domains of high electronic conductivity surrounding Si nanoparticles.34 Fig. 1b shows a scanning electron microscopy (SEM) image of the Si–pLig composite, with coated Si nanoparticles mostly in the size range of 110–170 nm diameter connected by the carbon network derived from pyrolized lignin. Kubo and Kadla found that the addition of up to 1% PEO to lignin blends greatly increased the thermal mobility and spinnability while decreasing the rigidity of lignin during either thermal extrusion or electrospinning.35,36 In our case, there is also a marked difference between Si–lignin composites with and without 0.5% PEO, as shown in Fig. 1c and S1,† respectively. Si–pLig with PEO appeared as uniform foam-like 3D network with voids, while without PEO Si nanoparticles were embedded in a rigid carbon matrix. Large fibers/spheres observed on the surface layer was identified to be carbon fibers/spheres from pyrolized lignin, likely because the surface conditions during DMF evaporation were similar to that in electrospinning from lignin/DMF solution which resulted in the formation of sphere/bead/fiber morphology.37 The morphological difference between the samples was also reflected in the electrochemical performance as shown later in this report. The Si–pLig composite electrode also showed excellent adhesion to Cu substrate even after 120 cycles, as shown from the cross section SEM image in Fig. S2a.†
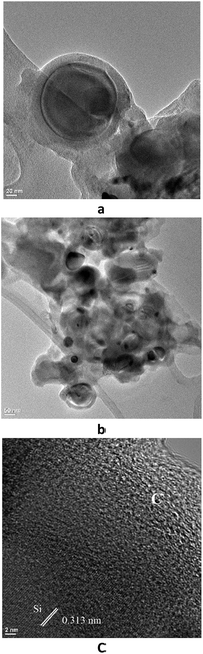
Transmission electron microscopy (TEM) images shown in Fig. 2a prove our hypothesis that Si nanoparticles were not only interconnected but also encapsulated by pyrolized lignin. A core–shell structure with 120 nm diameter Si nanoparticle and amorphous graphite coating of about 20 nm thickness were observed. Yolk–shell Si–pLig particles were also observed throughout the composite film (Fig. 2b). The observed yolk–shell particle formation in the composite may be explained by the fact that pyrolization char yield of hardwood kraft lignin is typically 50% leading to substantial lignin network volume reduction and contraction.26 It has been shown that the yolk–shell structure could greatly enhance capacity retention in Si-based lithium ion battery electrodes because it could better accommodate volume changes during lithiation and de-lithiation.38 The composite core–shell structure was further confirmed in Fig. 2c, where on the top right side an amorphous graphite layer of ∼20 nm thickness was observed, and on the bottom left side crystalline silicon was identified with a crystal lattice of 0.31 nm [(111) face].39
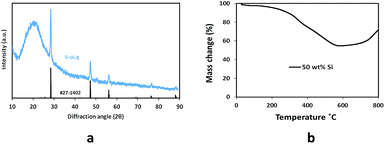
The structure of the Si–pLig composite was analyzed by X-ray diffraction (XRD) and shown in Fig. 3a. A broad peak between 15° and 25° can be attributed to amorphous carbon obtained from pyrolized lignin and possibly SiO2 amorphous peak upon comparison (JCPDS card number 29-0085). This shows that under a low temperature of 800 °C lignin was pyrolized primarily into an amorphous or hard carbon form.40 It can also be seen that silicon nanoparticles retained their crystallinity in the composite by comparison with crystalline silicon diffraction pattern (i.e., JCPDS #27-1402).
Thermogravimetric analysis (TGA) was performed to determine the weight percent of Si in the Si–pLig composite, shown in Fig. 3b. The initial monotonic mass decrease in the range of 400–600 °C was mainly due to oxidation of lignin and the mass increase after 600 °C due to oxidation of silicon in air, similar to previous reports.41,42 For a starting Si/lignin composite with Si nanoparticle weight composition of 50%, the resulting Si/SiOx wt% after pyrolysis was 54.7%. This was lower than the expected 66.7%, which is reasonable due to possibly incomplete pyrolization of lignin and the formation of thin SiOx layer at Si surface. In this report 50 wt% initial Si nanoparticle was chosen based on optimal results from previous publication, which also have shown that various Si/C ratios could be achieved by varying starting conditions, resulting in different specific capacities.41 This is one of the interesting areas we would like to study further in the future in order to optimize the performance of Si–pLig composite electrode.
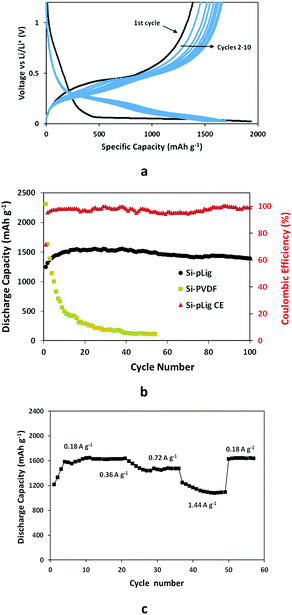
Fig. 4a shows the potential profile of the first 10 cycles of Si–pLig at a rate of 0.54 A g−1. The initial charge (lithiation) and discharge (delithiation) capacities for the composite were 1747 mA h g−1 and 1251 mA h g−1 respectively, with a coulombic efficiency (CE) of 71.6%. The irreversible capacity loss could be attributed to the formation of a SEI on the surface of the electrode at 0.6–0.9 V.43 Although the CE in the 1st cycle is unimpressive, discharge capacity increased with progressive cycling while the CE increased to 98.1% at cycle 10, likely due to surface activation or stabilization commonly observed in Si–C composite electrodes.44,45 The onset of the silicon lithiation potential long plateau at 0.12 V in the 1st cycle, which is different from cycles 2–10, is indicative of lithiation of crystalline silicon, which is understood because Si nanoparticles are crystalline as shown in TEM observations in Fig. 2c46 and XRD in Fig. 3a.
Long term cycling and rate performance of the Si–pLig composite electrode are shown in Fig. 4b. With the initial discharge capacity at 1251 mA h g−1 (Fig. S3†) it gradually increased over progressive cycling until reaching the highest capacity of 1557 mA h g−1 at 25th cycle. The discharge capacity at the 100th cycle is 1391 mA h g−1, a remarkable 89.3% capacity retention calculated against the highest capacity and an average cycling efficiency of 99.8% (Si–pLig CE) over 100 cycles. In contrast, the Si nanoparticle electrode with traditional PVDF binder as control (Si-PVDF) started at a high discharge capacity of 1732 mA h g−1, but quickly dropped to 25% at the 10th cycle and lost value during further cycling. Additionally, the Si–pLig composite electrode displayed excellent rate capabilities as shown in Fig. 4c. The average capacity based on the mass of Si is 1587 mA h g−1 at 0.18 A g−1 after initial stabilization, 1626 mA h g−1 at 0.36 A g−1, 1475 mA h g−1 at 0.72 A g−1, and 1133 mA h g−1 at 1.44 A g−1. When the current density is returned to 0.18 A g−1, the discharge capacity recovered to previous levels.
The exceptional electrochemical performance of the Si/C composite electrode may be understood based on the following considerations. First, carbon network obtained from pyrolized lignin acted as a mechanical support that encapsulated Si nanoparticles and provided good electrical conductivity; secondly, the encapsulation of carbon around Si nanoparticles allowed stable SEI formation and reduced irreversible reactions of active material with electrolyte; finally, carbon is known to be an excellent matrix for accommodating mechanical stress from volume changes in the lithiation/delithiation of Si, thus improving the cycling performance.5
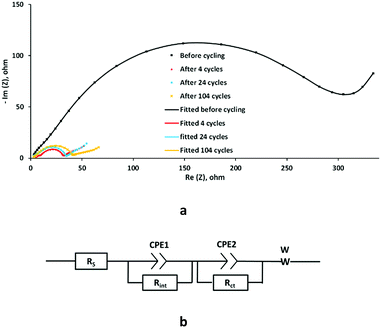
To study the mechanism of capacity loss during cycling, the electrochemical resistance of Si–pLig was measured by electrochemical impedance spectroscopy (EIS), with results shown in Fig. 5a. The impedance of the Si–pLig composite dropped dramatically after initial cycling which coincided with the capacity increase shown in Fig. 4a. This may be attributed to the activation process observed in Si–C composite materials.47 Although impedance slowly increased during cycling from cycle 4 to cycle 24 and cycle 104, the impedance of the Si–pLig composite was still smaller than it was before cycling. This indicates that the continuous capacity loss is not due to worsened kinetics but probably due to mechanical degradation and detachment of the composite, providing a direction for future improvement. The internal impedance of the cell could be described using the proposed equivalent circuit shown in Fig. 5b. In the equivalent circuit, RS is the resistance associated with the cell components such as electrolyte, the working electrode, and reference electrode. Rint is the interface resistance related to the SEI, Rct is the charge-transfer resistance, and W is the Warburg impedance element. Due to the porous structure of the Si–pLig composite electrode, the capacitor component in the equivalent circuit was replaced by the constant phase element.48 The experimental impedance values fitted well with the equivalent circuit.
Additionally, it is important to point out the effects of PEO addition during the composite electrode synthesis. As shown in Fig. S1,† without the 0.5 wt% PEO addition into the Si–Lig solution prior to pyrolization, Si precipitated from lignin matrix and a non-uniform composite electrode was formed. This expectedly resulted in poor electrochemical performance when it is compared to Si–pLig w 0.5% PEO, shown in Fig. S4.† As seen in Fig. S4,† discharge for Si–pLig w/o PEO started at 1.1 V and continued to 0.025 V, indicating rather significant SEI formation which deviated from typical discharge profiles of lithiation of crystalline silicon. This was also reflected in the low irreversible capacity of the first cycle, which we assigned to the poor contact between lignin and silicon without PEO to act as a “binder”. The capacity of the Si–pLig w PEO after the first charge/discharge cycle was approximately 7 times higher than the Si–pLig w/o PEO. In Fig. S4† the charge and discharge capacity of Si–pLig w/o PEO was shown over 100 cycles. The charge capacity of Si–pLig without PEO addition was less than 300 mA h g−1 in the first few cycles, much lower than the Si–pLig samples prepared with PEO, although it slightly increased over continuous cycling possibly due to activation mechanism discussed previously. Combined with the SEM images, we conclude that the addition of 0.5 wt% PEO (1 × 106 molecular weight (MW)) was crucial to form a backbone framework for the lower molecular weight lignin (average MW 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–30
000–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and eventual formation of foam-like Si–pLig composite. Without the addition of 0.5 wt% PEO low molecular weight lignin molecules were unable to uniformly coat Si particles prior to and during pyrolization, which resulted in detrimental SEI formation on Si nanoparticles. Judging from the low discharge and charge capacity contribution from Si we believe that many Si nanoparticles were disconnected from the carbon matrix. The beneficial effect of PEO on improving spinnability of lignin into carbon fiber network was studied by Kadla et al., which could also be used to explain the present phenomenon since in both cases the desire was to form an ordered interconnected morphology.26,37,49 We believe two reasons that may account for the marked difference: (1) PEO is known to greatly promote hydrogen bonding along with other intermolecular and intra-molecular forces between lignin blends thus increasing the viscosity of Si–Lig composite which helps maintain the microstructure during heat treatment. (2) Enhanced Si nanoparticle–lignin hydrogen bonding interactions allow for better encapsulation of Si nanoparticles.50
000) and eventual formation of foam-like Si–pLig composite. Without the addition of 0.5 wt% PEO low molecular weight lignin molecules were unable to uniformly coat Si particles prior to and during pyrolization, which resulted in detrimental SEI formation on Si nanoparticles. Judging from the low discharge and charge capacity contribution from Si we believe that many Si nanoparticles were disconnected from the carbon matrix. The beneficial effect of PEO on improving spinnability of lignin into carbon fiber network was studied by Kadla et al., which could also be used to explain the present phenomenon since in both cases the desire was to form an ordered interconnected morphology.26,37,49 We believe two reasons that may account for the marked difference: (1) PEO is known to greatly promote hydrogen bonding along with other intermolecular and intra-molecular forces between lignin blends thus increasing the viscosity of Si–Lig composite which helps maintain the microstructure during heat treatment. (2) Enhanced Si nanoparticle–lignin hydrogen bonding interactions allow for better encapsulation of Si nanoparticles.50
Conclusions
We have demonstrated that low-cost and renewable lignin could be composited with Si nanoparticles to form electrodes that exhibit high specific capacity during lithium charge/discharge cycles, with performance comparable to some of the recent publications on silicon nanoparticle electrodes.8,21,51–53Our current work is important in two aspects: (1) the use of lignin as a renewable and low cost precursor for fabricating high performance Si electrodes for lithium ion batteries; (2) pyrolized lignin with PEO as a backbone forms a binder free matrix with excellent electronic conductivity, ionic conductivity, and adhesion, thus removing the need for conventional binders such as PVDF. Although lignin has been previously explored for use in sodium ion batteries54 and spun into carbon fibers (CF) as active material/support for lithium ion batteries,32,55,56 this is, to the best of our knowledge, one of the first reports in using lignin as a precursor to form Si–C composites for lithium ion batteries. There are variables not explored in this current work which may further enhance the performance of Si–pLig composite electrodes, such as optimizing Si–Lig ratio, Si nanoparticle size, pyrolization temperature/time, and PEO concentration/weight percent. We believe that the low cost, renewability, and environmental friendliness associated with lignin and cost reduction by omitting binders will stimulate more interest in utilizing lignin and other biopolymers in energy related research.
Acknowledgements
We acknowledge the support from National Science Foundation Grant No. 1355438 (Powering the Kentucky Bio-economy for a Sustainable Future).Notes and references
- B. Kang and G. Ceder, Nature, 2009, 458, 190–193 CrossRef CAS PubMed.
- T. D. Hatchard and J. R. Dahn, J. Electrochem. Soc., 2004, 151, A838–A842 CrossRef CAS.
- L. Y. Beaulieu, K. W. Eberman, R. L. Turner, L. J. Krause and J. R. Dahn, Electrochem. Solid-State Lett., 2001, 4, A137–A140 CrossRef CAS.
- I. Kim, G. E. Blomgren and P. N. Kumta, Electrochem. Solid-State Lett., 2003, 6, A157–A161 CrossRef CAS.
- U. Kasavajjula, C. S. Wang and A. J. Appleby, J. Power Sources, 2007, 163, 1003–1039 CrossRef CAS.
- A. M. Wilson, J. N. Reimers, E. W. Fuller and J. R. Dahn, Solid State Ionics, 1994, 74, 249–254 CrossRef CAS.
- C. Q. Zhang, F. Yang, D. L. Zhang, X. Zhang, C. L. Xue, Y. H. Zuo, C. B. Li, B. W. Cheng and Q. M. Wang, RSC Adv., 2015, 5, 15940–15943 RSC.
- J. Li, R. B. Lewis and J. R. Dahn, Electrochem. Solid-State Lett., 2007, 10, A17–A20 CrossRef CAS.
- I. Kovalenko, B. Zdyrko, A. Magasinski, B. Hertzberg, Z. Milicev, R. Burtovyy, I. Luzinov and G. Yushin, Science, 2011, 334, 75–79 CrossRef CAS PubMed.
- J. Xu, Q. Zhang and Y.-T. Cheng, J. Electrochem. Soc., 2016, 163, A401–A405 CrossRef CAS.
- J. Graetz, C. C. Ahn, R. Yazami and B. Fultz, Electrochem. Solid-State Lett., 2003, 6, A194–A197 CrossRef CAS.
- J. P. Maranchi, A. F. Hepp and P. N. Kumta, Electrochem. Solid-State Lett., 2003, 6, A198–A201 CrossRef CAS.
- Q. L. Zhang, X. C. Xiao, W. D. Zhou, Y. T. Cheng and M. W. Verbrugge, Adv. Energy Mater., 2015, 5 Search PubMed.
- J. R. Szczech and S. Jin, Energy Environ. Sci., 2011, 4, 56–72 CAS.
- H. Ma, F. Y. Cheng, J. Chen, J. Z. Zhao, C. S. Li, Z. L. Tao and J. Liang, Adv. Mater., 2007, 19, 4067–4070 CrossRef CAS.
- C. K. Chan, H. L. Peng, G. Liu, K. McIlwrath, X. F. Zhang, R. A. Huggins and Y. Cui, Nat. Nanotechnol., 2008, 3, 31–35 CrossRef CAS PubMed.
- L. F. Cui, Y. Yang, C. M. Hsu and Y. Cui, Nano Lett., 2009, 9, 3370–3374 CrossRef CAS PubMed.
- M. H. Park, M. G. Kim, J. Joo, K. Kim, J. Kim, S. Ahn, Y. Cui and J. Cho, Nano Lett., 2009, 9, 3844–3847 CrossRef CAS PubMed.
- Y. J. Chen, M. Y. Nie, B. L. Lucht, A. Saha, P. R. Guduru and A. Bose, ACS Appl. Mater. Interfaces, 2014, 6, 4678–4683 CAS.
- X. C. Xiao, W. D. Zhou, Y. N. Kim, I. Ryu, M. Gu, C. M. Wang, G. Liu, Z. Y. Liu and H. J. Gao, Adv. Funct. Mater., 2015, 25, 1426–1433 CrossRef CAS.
- H. Wu, G. H. Yu, L. J. Pan, N. A. Liu, M. T. McDowell, Z. A. Bao and Y. Cui, Nat. Commun., 2013, 4 CAS.
- Y. Yao, M. T. McDowell, I. Ryu, H. Wu, N. A. Liu, L. B. Hu, W. D. Nix and Y. Cui, Nano Lett., 2011, 11, 2949–2954 CrossRef CAS PubMed.
- M. W. Verbrugge, D. R. Baker, X. Xiao, Q. Zhang and Y.-T. Cheng, J. Phys. Chem. C, 2015, 119, 5341–5349 CAS.
- W. Boerjan, J. Ralph and M. Baucher, Annu. Rev. Plant Biol., 2003, 54, 519–546 CrossRef CAS PubMed.
- S. Chatterjee, E. B. Jones, A. C. Clingenpeel, A. M. McKenna, O. Rios, N. W. McNutt, D. J. Keffer and A. Johs, ACS Sustainable Chem. Eng., 2014, 2, 2002–2010 CrossRef CAS.
- J. F. Kadla, S. Kubo, R. A. Venditti, R. D. Gilbert, A. L. Compere and W. Griffith, Carbon, 2002, 40, 2913–2920 CrossRef CAS.
- M. Lallave, J. Bedia, R. Ruiz-Rosas, J. Rodriguez-Mirasol, T. Cordero, J. C. Otero, M. Marquez, A. Barrero and I. G. Loscertales, Adv. Mater., 2007, 19, 4292–4296 CrossRef CAS.
- R. Ruiz-Rosas, J. Bedia, M. Lallave, I. G. Loscertales, A. Barrero, J. Rodriguez-Mirasol and T. Cordero, Carbon, 2010, 48, 696–705 CrossRef CAS.
- J. Lin, K. Koda, S. Kubo, T. Yamada, M. Enoki and Y. Uraki, J. Wood Chem. Technol., 2014, 34, 111–121 CrossRef CAS.
- V. Dave, A. Prasad, H. Marand and W. G. Glasser, Polymer, 1993, 34, 3144–3154 CrossRef CAS.
- G. Milczarek and O. Inganas, Science, 2012, 335, 1468–1471 CrossRef CAS PubMed.
- W. E. Tenhaeff, O. Rios, K. More and M. A. McGuire, Adv. Funct. Mater., 2014, 24, 86–94 CrossRef CAS.
- J. Jin, S. J. Yu, Z. Q. Shi, C. Y. Wang and C. B. Chong, J. Power Sources, 2014, 272, 800–807 CrossRef CAS.
- H. P. Yang, R. Yan, H. P. Chen, D. H. Lee and C. G. Zheng, Fuel, 2007, 86, 1781–1788 CrossRef CAS.
- S. Kubo and J. F. Kadla, J. Appl. Polym. Sci., 2005, 98, 1437–1444 CrossRef CAS.
- I. Dallmeyer, F. Ko and J. F. Kadla, Ind. Eng. Chem. Res., 2014, 53, 2697–2705 CrossRef CAS.
- I. Dallmeyer, F. Ko and J. F. Kadla, J. Wood Chem. Technol., 2010, 30, 315–329 CrossRef CAS.
- R. K. Sharma, J. B. Wooten, V. L. Baliga, X. H. Lin, W. G. Chan and M. R. Hajaligol, Fuel, 2004, 83, 1469–1482 CrossRef CAS.
- K. Chen, Z. H. Bao, J. Shen, G. M. Wu, B. Zhou and K. H. Sandhage, J. Mater. Chem., 2012, 22, 16196–16200 RSC.
- J. Seo, H. Park, K. Shin, S. H. Baeck, Y. Rhym and S. E. Shim, Carbon, 2014, 76, 357–367 CrossRef CAS.
- T. H. Hwang, Y. M. Lee, B. S. Kong, J. S. Seo and J. W. Choi, Nano Lett., 2012, 12, 802–807 CrossRef CAS PubMed.
- J. C. Guo, X. L. Chen and C. S. Wang, J. Mater. Chem., 2010, 20, 5035–5040 RSC.
- M. Li, X. H. Hou, Y. J. Sha, J. Wang, S. J. Hu, X. Liu and Z. P. Shao, J. Power Sources, 2014, 248, 721–728 CrossRef CAS.
- L. Zhou, X. J. Lin, T. Huang and A. S. Yu, Electrochim. Acta, 2014, 116, 210–216 CrossRef CAS.
- J. Yang, B. F. Wang, K. Wang, Y. Liu, J. Y. Xie and Z. S. Wen, Electrochem. Solid-State Lett., 2003, 6, A154–A156 CrossRef CAS.
- M. N. Obrovac and L. Christensen, Electrochem. Solid-State Lett., 2004, 7, A93–A96 CrossRef CAS.
- X. L. Wang, G. Li, F. M. Hassan, M. Li, K. Feng, X. C. Xiao and Z. W. Chen, J. Mater. Chem. A, 2015, 3, 3962–3967 CAS.
- D. Aurbach, M. D. Levi, E. Levi, H. Teller, B. Markovsky, G. Salitra, U. Heider and L. Heider, J. Electrochem. Soc., 1998, 145, 3024–3034 CrossRef CAS.
- I. Dallmeyer, L. T. Lin, Y. J. Li, F. Ko and J. F. Kadla, Macromol. Mater. Eng., 2014, 299, 540–551 CrossRef CAS.
- D. Munao, J. W. M. van Erven, M. Valvo, E. Garcia-Tamayo and E. M. Kelder, J. Power Sources, 2011, 196, 6695–6702 CrossRef CAS.
- H. Wang, Z. W. Xu, A. Kohandehghan, Z. Li, K. Cui, X. H. Tan, T. J. Stephenson, C. K. King'ondu, C. M. B. Holt, B. C. Olsen, J. K. Tak, D. Harfield, A. O. Anyia and D. Mitlin, ACS Nano, 2013, 7, 5131–5141 CrossRef CAS PubMed.
- X. L. Chen, X. L. Li, F. Ding, W. Xu, J. Xiao, Y. L. Cao, P. Meduri, J. Liu, G. L. Graff and J. G. Zhang, Nano Lett., 2012, 12, 4124–4130 CrossRef CAS PubMed.
- Y. S. Hu, R. Demir-Cakan, M. M. Titirici, J. O. Muller, R. Schlogl, M. Antonietti and J. Maier, Angew. Chem., Int. Ed., 2008, 47, 1645–1649 CrossRef CAS PubMed.
- J. Jin, B.-j. Yu, Z.-q. Shi, C.-y. Wang and C.-b. Chong, J. Power Sources, 2014, 272, 800–807 CrossRef CAS.
- S. X. Wang, L. P. Yang, L. P. Stubbs, X. Li and C. B. He, ACS Appl. Mater. Interfaces, 2013, 5, 12275–12282 CAS.
- O. Rios, S. K. Martha, M. A. McGuire, W. Tenhaeff, K. More, C. Daniel and J. Nanda, Energy Technol., 2014, 2, 773–777 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and supplementary figures are available. See DOI: 10.1039/c6ra03001g |
| This journal is © The Royal Society of Chemistry 2016 |