Nickel(ii)-2-amino-4-alkoxy-1,3,5-triazapentadienate complexes as catalysts for Heck and Henry reactions†
Abstract
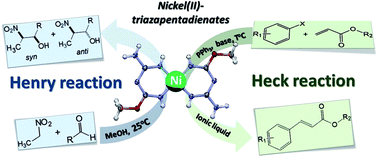
Nickel(II)-triazapentadienate complexes, [Ni(tap)2], are synthesized and found to act as catalysts in the Heck reaction of deactivated aryl halides. The use of room-temperature ionic liquids instead of organic solvents allows easy separation of the catalyst from the products and substrates. The synthesized complexes also catalyse the Henry reaction of benzaldehydes with nitroethane, at 25 °C, leading to the corresponding nitroalkanols with diastereoselectivity in favour of the anti isomer.


 Please wait while we load your content...
Please wait while we load your content...