Nitrogen-doped TiO2 microspheres with hierarchical micro/nanostructures and rich dual-phase junctions for enhanced photocatalytic activity†
Wei Wanga,
Yu Liua,
Jifa Qub,
Yubo Chenb and
Zongping Shao*ab
aDepartment of Chemical Engineering, Curtin University, Perth, WA 6845, Australia. E-mail: zongping.shao@curtin.edu.au
bState Key Laboratory of Materials-Oriented Chemical Engineering, Nanjing Tech University, Nanjing, 210009, China
First published on 11th April 2016
Abstract
We successfully synthesized microspherical nitrogen-doped TiO2 with hierarchical nano/microstructures, rich anatase TiO2–TiO2(B) phase junctions and a reduced band gap by a facile solvothermal process, followed by a urea-based solid-state reaction. Three kinds of nitrogen species in different doping sites, which can improve the photocatalytic activity and reduce the band gap, were found in the hierarchical nitrogen-doped TiO2 microspheres. In particular, a suitable amount of nitrogen doping was found to effectively reduce the Ti3+ concentration in TiO2, thus benefiting the photocatalytic reaction by reducing the recombination centers in the photocatalyst. The combination of hierarchical microspherical morphology, the rich phase junctions and narrowed band gap gives nitrogen-doped TiO2 microspheres remarkably improved photocatalytic activity, compared with commercial mixed-phase TiO2 (P25) and single-phase anatase-type TiO2. Furthermore, nitrogen-doped TiO2 microspheres display reliable photocatalytic performance for multiple cycles. The as-prepared nitrogen-doped TiO2 microspheres hold promise as efficient photocatalysts for various photocatalytic applications.
Introduction
The capture and conversion of solar energy by semiconductor-based heterogeneous photocatalysis has gained significant attention from the scientific and engineering world because of the great potential for resolving energy and environmental issues toward sustainable processes; for example, photocatalytic water splitting, photodegradation of organic pollutants and photoreduction of CO2 to solar fuels.1–3 The efficiency of photocatalysis strongly relies on the catalyst itself, which should be well developed and designed.4–6 Among various photocatalysts, titanium dioxide (TiO2) has been shown to be promising due to its effective oxidizing power, good durability and low cost.7–10 However, pristine TiO2 is only photoactive in UV light, due to its wide band gap (3.2 eV), which limits the efficient utilization of sunlight because the UV light only captures approximately 5% of all solar energy. The question of how to utilize the visible portion of the solar spectrum, which contains >45% of solar energy, has become a great concern for the development of TiO2-based photocatalysts and thus, it is of great importance to reduce the band gap of TiO2.The band gap of semiconductor materials can be reduced using a doping strategy in which the effective electronegativities of the compositional cations and the anion are controlled.11–17 Some metal elements such as Ag, Fe and Mo, with electronegativities larger than Ti but closer to O, have been used as dopants for TiO2 to reduce the band gap.12–14 In addition, non-metal element (N, S and C) doping is also regarded as an effective way to reduce the band gap of TiO2 because these elements have an electronegativity smaller than O but close to Ti.15–17 Non-metal element doping is more favorable because cation doping generates more recombination centers for the photo-generated charge carriers, which are harmful to photocatalytic reactions. Among the various non-metal element dopants for TiO2, nitrogen is the most investigated dopant because it provides the most significant improvement in the visible light response of TiO2.18–23 Besides the band gap, the high degree of recombination of photo-generated electrons and holes is a major limiting factor that controls photocatalytic efficiency.11,24–26 Therefore, a major challenge in heterogeneous photocatalysis is to suppress the recombination of photo-generated electron–hole pairs in the photocatalysts. The recombination of electrons and holes is closely related to the morphology/microstructure of TiO2, which in turn is strongly affected by the preparation method.7,8,27,28 The microstructure may also affect the charge/mass transfer within the catalyst and consequently, the photocatalytic activity. Thus, a rational design of the morphological structure of TiO2 is also crucial to achieving high photocatalytic activity.
In addition to doping and morphological tailoring, it has been reported that the creation of phase junctions in TiO2 (such as anatase and rutile phases) is also an effective way to improve photocatalytic activity.29,30 Photocatalysts composed of mixed TiO2 phases have attracted more and more attention recently, as they display much higher catalytic activity than either of the single-phase components. There are two factors that can strongly affect the charge transfer from the phase possessing the higher conduction band edge to the other phase.30 The first is the interface structure, which is crucial to the charge transfer. Defects, large crystallographic discrepancies and voids at the interface may form charge traps and will hinder the charge transfer in the photocatalysts with mixed TiO2 phases. The second factor is the mobility of the photo-generated charges, as the excited electrons migrate much slower than the holes. This factor also affects the migration of the charges to the surface. Furthermore, the pathways are not independent but are interrelated.30 For example, molecular oxygen on the TiO2 surface can capture the excited CB electrons through the formation of superoxide ions, which can subsequently transform into other active chemical species, reducing the recombination of electron–hole pairs.30 Taking the TiO2(B) and anatase phase-junctions as an example, the difference between the band edges of the two phases can promote the charge transfer from anatase TiO2 to TiO2(B); the well-matched phase interfaces make it possible for the photo-generated holes to migrate from anatase TiO2 to TiO2(B).30 Both the interphase transfer of the holes and the electron capturing on the surface contribute to reducing the recombination of the photo-generated charges, and enhance photocatalytic activity.30 This suggests that the interfaces of mixed TiO2 phases play a crucial role in the achievement of high photocatalytic activity.
Considering the fact that the photocatalytic activity of TiO2 is affected by so many parameters, optimization from any one single point may not be an efficient way to maximize the photocatalytic performance of TiO2. Thus, full-scale tailoring of the properties of TiO2 from different aspects is highly desirable. The simultaneous nitrogen doping, morphological control and introduction of phase junctions in TiO2 may effectively extend the photoactive region, reduce the recombination rate of electrons and holes and increase the charge/mass transfer, thereby effectively enhancing the photocatalytic performance.
Herein, we successfully synthesized a microspherical nitrogen-doped TiO2 with hierarchical micro/nanostructures, reduced band gap and rich anatase TiO2–TiO2(B) phase junctions for significantly enhanced photocatalytic activity. We used a facile solvothermal approach and a subsequent solid-state reaction with urea to synthesize this nitrogen-doped TiO2. As expected, the as-prepared nitrogen-doped TiO2 demonstrates much higher photocatalytic activity than commercial P25. In addition, the specific microspherical structure of nitrogen doped TiO2 helps the homogenous dispersion of the catalyst in water using electrostatic surface charges, without the need for additional capping ligands. Furthermore, the microspherical structure enables easy recycling by filtration or sedimentation, making it a highly promising photocatalyst with great potential for various photocatalytic applications.
Experimental
All chemicals were of analytical grade and were used in this study without further purification. The synthesis of hierarchical porous TiO2 microspheres was performed by a solvothermal process. Typically, 1 mL of tetrabutyl titanate (TBT) was added dropwise to 50 mL of a polyethylene pyrrole (PVP, K-30) (0.24 g) and acetic acid solution with continuous stirring for 5 min. The obtained white suspension was transferred to a 100 mL Teflon-lined stainless-steel autoclave. After reacting at 150 °C for 24 h, the product was washed with an ethanol–water mixture several times, centrifuged at 4000 rpm, dried at 80 °C and then calcined at 400 °C for 2 h in air to obtain the porous TiO2 microspheres. Different TiO2 to urea ratios were used to incorporate nitrogen including 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and 1
8 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 for N–TiO2 (1–4), N–TiO2 (1–8) and N–TiO2 (1–16), respectively. Taking the ratio of 1
16 for N–TiO2 (1–4), N–TiO2 (1–8) and N–TiO2 (1–16), respectively. Taking the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 as an example, 0.2 g of the TiO2 microspheres were mixed with 0.8 g of urea and then heated in a covered crucible at 400 °C in air for 3 h.
4 as an example, 0.2 g of the TiO2 microspheres were mixed with 0.8 g of urea and then heated in a covered crucible at 400 °C in air for 3 h.
The photocatalytic activities of TiO2 and nitrogen-doped TiO2 samples were evaluated by a model photocatalytic reaction (degradation of methyl orange, MO). A metal halide lamp (575 W, Philips) was utilized as the radiation source. In a typical process, 200 mL of a 10 mg L−1 (10 ppm) MO solution were mixed with 0.1 g of photocatalyst and continuously stirred in a double-jacket reactor, which was then irradiated by UV-visible light without a filter. The reaction temperature was maintained at 25 °C by flowing cooled water. During the light irradiation, approximately 10 mL of suspension were collected every 30 min. After the removal of the photocatalyst particles from the suspension by a millipore filter with pore size of 0.45 μm (FilterBio MCE Membrane Filter), the obtained solution was analyzed with a UV-Vis spectrometer at 463 nm. The Lambert–Beer rule was applied for an absorbance band characteristic of the dye to determine its concentration. P25 (DeGussa) was a typical reference TiO2 for the evaluation of the photocatalytic activity of the nitrogen-doped TiO2 microspheres and other TiO2-based photocatalysts.
Powder XRD patterns were examined on a Bruker D8 Advance X-ray diffractometer using Cu Kα radiation with a range of 10–80° (2θ) with intervals of 0.02°. Rietveld refinement of XRD patterns was obtained using the GSAS-EXPGUI software. SEM images were obtained on a JEOL S4800 instrument with an accelerating voltage of 5.0 kV. XPS measurements were performed on a Thermo ESCALAB 250 using monochromatic Al Kα radiation (1486.6 eV). All binding energies were referenced to the C 1s peak at 283.6 eV, and experimental errors were within ±0.1 eV. The morphology and microstructure of nitrogen-doped TiO2 were investigated by TEM (JEOL 2100). Brunauer–Emmett–Teller (BET) specific surface areas and pore size distributions were obtained using adsorption data from a Micromeritics TriStar II instrument. Fourier transform infrared spectroscopy (FTIR) was performed on a Perkin-Elmer Model FTIR-100 with an MIR detector. UV-Vis DRS of samples were recorded on a JASCO V670 spectrophotometer with a Φ60 mm integrating sphere. BaSO4 was used as a reference material. The Raman spectra were obtained in an HR800 UV Raman microspectrometer (JOBIN YVON, France). Photoluminescence (PL) spectra were obtained in PerkinElmer luminescence spectrometer.
Results and discussion
Fig. 1 shows the X-ray diffraction (XRD) patterns of the as-prepared pristine TiO2 microspheres and the various nitrogen-doped TiO2 microspheres with different amounts of urea in the synthesis (N–TiO2 (1–x), where x represents the ratio of urea to TiO2 during the synthesis). The XRD pattern of commercial P25, which is composed of anatase and rutile TiO2 mixed phases, is also presented as a reference. The main diffraction peaks of the as-prepared TiO2 and the nitrogen-doped TiO2, which were both calcined at 400 °C, can be well indexed as a mixture of anatase TiO2 and TiO2(B) phases, as shown in Fig. 1. This suggests that anatase TiO2–TiO2(B) phase junctions may be present in both samples. To obtain information about the exact phase compositions, XRD patterns of the as-prepared TiO2 and N–TiO2 (1–8) samples were subjected to Rietveld refinements (Fig. S1a and b†). The derived weight percentages of the different phases in both samples, and their reliability factors are listed in Table S1.† The pristine TiO2 was composed of 88 wt% anatase TiO2 and 12 wt% TiO2(B), whereas the N–TiO2 (1–8) sample consisted of 82 wt% anatase TiO2 and 18 wt% TiO2(B). This indicates that nitrogen doping did not have a big impact on the phase structure of TiO2. | ||
| Fig. 1 XRD patterns of P25, the pristine TiO2 and various nitrogen-doped TiO2 samples with different urea to TiO2 ratios. | ||
Raman spectra were further investigated to confirm the mixed phases of anatase and TiO2(B) in the pristine TiO2 and N–TiO2 (1–8) microspheres and the results are shown in Fig. S2.† The Raman peaks at 395, 516 and 639 cm−1 are typical Raman features of the anatase TiO2 phase. Although most of the peaks of anatase TiO2 were superimposed with the peaks of TiO2(B), the formation of anatase TiO2 was confirmed by the characteristic anatase peak at 395 cm−1, which did not overlap with any peaks of TiO2(B). The other peaks (except at 395 cm−1) can also be indexed as the vibration modes of the TiO2(B) phase, which was consistent with the XRD results. The appearance of characteristic peaks of TiO2(B) at 235 cm−1 indicates that the pristine TiO2 and N–TiO2 (1–8) microspheres were composed of mixed TiO2 phases. As shown in Fig. S3,† the peaks of pristine TiO2 and N–TiO2 (1–8) in the PL spectra were around 435 nm, also suggesting the mixed anatase and TiO2(B) phases, since the peaks of anatase and TiO2(B) in the PL spectra were reported to be 450 and 425 nm, respectively.31,32
The particulate morphologies of the as-prepared TiO2 and the various nitrogen-doped TiO2 samples were first examined by scanning electron microscopy (SEM), with representative images shown in Fig. 2. All of the samples are composed of mono-disperse spherical particles, approximately 2–3 μm in diameter; however, this special spherical structure was partially destroyed in the N–TiO2 (1–16) sample, which was likely caused by the large amount of gas-phase products from the urea decomposition during calcination. Fig. 3 presents high magnification SEM images of the microsphere morphology in the pristine TiO2 and N–TiO2 (1–8) samples. Such microspheres were actually built from many belt-shaped nanostructures, as shown in Fig. 3e, the existence of which was confirmed by our previous research.33 No obvious difference in particulate morphology between the TiO2 and N–TiO2 (1–8) samples was observed. This suggests that the proper amount of nitrogen doping did not greatly influence the particulate morphology of the TiO2 samples.
The phase composition and particulate morphology of the as-prepared samples were further examined by transmission electron microscopy (TEM) and high resolution (HR)-TEM. As shown in Fig. 4a and b, the TiO2 microsphere was assembled from many porous TiO2 nanosheets, with length of approximately 200 nm and thickness of 30–50 nm. These leaf-like nanobelts grew from the center and combined to produce well-organized porous microspheres with a highly open structure. The HR-TEM images demonstrate the well-crystallized and highly mesoporous nature of the nanostructures, which are composed of TiO2 nanocrystallites. Both anatase TiO2 and TiO2(B) phases are present in the nanocrystallites, confirmed by the fact that clear fringe planes corresponding to both phases were observed in the same leaf. For example, the (220) and (310) fringe planes of TiO2(B) and the (103) and (202) fringe planes of anatase TiO2 are shown in Fig. 4c, and the (311) fringe plane of TiO2(B) and the (105) fringe plane of anatase TiO2 are shown in Fig. 4d. Furthermore, the anatase TiO2 phase and the TiO2(B) phase are well connected to each other and likely form rich phase junctions inside the N–TiO2 (1–8) sample. It has been reported that the heterojunction interface formed between anatase TiO2 and TiO2(B) can promote charge migration, which benefits the photocatalytic reaction.30
 | ||
| Fig. 4 TEM images of (a) a N–TiO2 (1–8) microsphere, (b) the nanosheets at the surface of a microsphere. (c and d) HR-TEM images of the nanosheet with two different phase structures. | ||
Fig. 5 shows the nitrogen adsorption–desorption isotherms and the corresponding Barrett–Joyner–Halenda (BJH) pore size distributions obtained for the various samples. The specific surface areas of the samples calculated from the nitrogen adsorption–desorption isotherms in Fig. 5a are 150.3, 128.0, 116.3 and 112.5 m2 g−1 for pristine TiO2, N–TiO2 (1–4), N–TiO2 (1–8) and N–TiO2 (1–16), respectively. These data indicate that the reaction with urea slightly reduced the specific surface areas of the TiO2 samples. As shown in Fig. 5b, there are two kinds of mesopores (with average pore sizes of ∼2.2 and ∼8.0 nm) in pristine TiO2, whereas N–TiO2 (1–16) displays a pore size distribution that is different from the other three catalysts, in which the amount of the smaller pores increased sharply, and the larger pores almost disappeared (Fig. 5b). Such a difference may be correlated with the morphological change in the microspheres in the N–TiO2 (1–16) sample due to the overuse of urea during the synthesis.
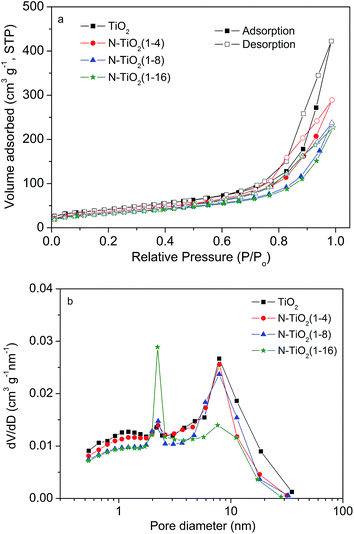 | ||
| Fig. 5 (a) Nitrogen adsorption/desorption isotherm patterns and (b) BJH pore size distribution curves of the as-prepared pristine TiO2 and TiO2 doped with various amounts of nitrogen. | ||
To confirm the successful nitrogen doping of TiO2, FTIR was conducted. The typical spectra of pristine TiO2 and the various N–TiO2 samples are shown in Fig. S4.† All four samples show similar FTIR spectra, in which the bands located from 600 to 850 cm−1 were attributed to Ti–O stretching and Ti–O–Ti bridging stretching modes.34 The band at approximately 3400 cm−1 was indexed to the stretching vibration of the O–H bond and free water, whereas the band at approximately 1631 cm−1 was attributed to the O–H bending vibration of chemically adsorbed water.34,35 It was found that the incorporation of nitrogen into the oxide lattice increased the amount of hydroxyl groups and adsorbed water molecules on the surface of the catalysts. Hydroxyl groups and adsorbed water molecules could act as hole traps and produce hydroxyl radicals for the degradation of phenol molecules and dyes in water solutions.34,35 Fig. S4b† shows the localized profile of the FTIR spectra within a range of 950 to 1550 cm−1. The bands at approximately 1474, 1250 and 1080 cm−1 are attributed to the vibrations of the Ti–N bond, whereas the band at 1160 cm−1 is ascribed to the nitrite peak.36 In addition, the band for the bending mode of the N–H bond at 1411 cm−1 and the band for NOx at approximately 1318 cm−1 were also identified. No such nitrogen-related bands were observed in the pristine TiO2.37,38 All of the above information further confirmed the successful incorporation of different nitrogen species into TiO2 as a consequence of being calcined with urea.
The chemical components at the surface of the various samples were analyzed by X-ray photoelectron spectra (XPS). Nitrogen doping in TiO2 can result in various nitrogen species such as substitutional nitrogen (Ns), interstitial nitrogen (Ni) and NOx species.39 The NOx species are most likely present on the surface of TiO2 or trapped in the voids of the solids,40 but the Ni and Ns species are probably present in subsurface layers.41 The N 1s XPS spectra for the N–TiO2 (1–8) sample are shown in Fig. 6a, and the broad peak can be fitted by three peaks at 405.9, 400.1 and 398.4 eV, suggesting the presence of three independent environments for the nitrogen. The low binding energy (BE) component located at 398.5 eV is generally known as Ns and forms an O–Ti–N bond in the TiO2 crystal lattice.36,42 The peak at 400.1 eV can be attributed to the nitrogen in Ti–O–N bonding, which could be assigned to Ni. The peak with a BE of approximately 405.9 eV can be assigned to NOx species, such as NO or NO2. The ratio of Ns to Ni in the N–TiO2 (1–8) sample is approximately 2.12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, according to the area ratio of N 1s peaks as shown in Fig. 6a. Some researchers have used theoretical calculations and experimental studies to demonstrate that the Ns species can generate states above the valence band maximum (VBM). Although these states can mix with O 2p valence states to reduce the band gap of TiO2, no band gap narrowing was obtained by calculations for the Ni species in the form of Ti–O–N, as can be detected by XPS.39,43,44 Furthermore, Peng et al. reported that the photocatalytic activity of interstitial nitrogen-doped TiO2 is higher than that of substitutional nitrogen-doped TiO2.45 It was found that the combination of Ns and Ni is beneficial for the achievement of a reduced band gap and higher photocatalytic activity. Recently, Lin et al. reported a nitrogen-doped TiO2 with Ns and Ni species that displayed excellent photocatalytic activity, as well as a smaller band gap, although the ratio of Ns to Ni was different from the results in this study.23 On the other hand, it was found that the enhanced visible light response by nitrogen doping may not be due solely to Ns or Ni; NOx species can also contribute to visible light absorption because they also generate intragap states like Ns or Ni.39 Therefore, high photocatalytic activity should be expected because three different nitrogen species were incorporated together in the N–TiO2 (1–8) sample.
1, according to the area ratio of N 1s peaks as shown in Fig. 6a. Some researchers have used theoretical calculations and experimental studies to demonstrate that the Ns species can generate states above the valence band maximum (VBM). Although these states can mix with O 2p valence states to reduce the band gap of TiO2, no band gap narrowing was obtained by calculations for the Ni species in the form of Ti–O–N, as can be detected by XPS.39,43,44 Furthermore, Peng et al. reported that the photocatalytic activity of interstitial nitrogen-doped TiO2 is higher than that of substitutional nitrogen-doped TiO2.45 It was found that the combination of Ns and Ni is beneficial for the achievement of a reduced band gap and higher photocatalytic activity. Recently, Lin et al. reported a nitrogen-doped TiO2 with Ns and Ni species that displayed excellent photocatalytic activity, as well as a smaller band gap, although the ratio of Ns to Ni was different from the results in this study.23 On the other hand, it was found that the enhanced visible light response by nitrogen doping may not be due solely to Ns or Ni; NOx species can also contribute to visible light absorption because they also generate intragap states like Ns or Ni.39 Therefore, high photocatalytic activity should be expected because three different nitrogen species were incorporated together in the N–TiO2 (1–8) sample.
 | ||
| Fig. 6 XPS spectra of (a) N 1s for N–TiO2 (1–8), (b) O 1s for pure TiO2 and N–TiO2 (1–8), and (c) Ti 2p for pure TiO2 and N–TiO2 (1–8). | ||
Fig. 6b shows O 1s XPS spectra of the pristine TiO2 and N–TiO2 (1–8) samples. Two kinds of oxygen were found to exist in the N–TiO2 (1–8) and pristine TiO2 samples, and the ratio of the absorbed oxygen (531.1 eV) to lattice oxygen (529.6 eV) increased after nitrogen doping, which is in good agreement with the literature.46 In addition, the substitution of lattice oxygen by the nitrogen anion led to a decrease in the amount of lattice oxygen, which further confirmed the successful doping of nitrogen in the TiO2 lattice of the N–TiO2 (1–8) sample. Fig. 6c shows the Ti 2p XPS spectra of both the as-prepared pristine TiO2 and N–TiO2 (1–8) samples. Two main peaks were observed and assigned to Ti 2p1/2 and Ti 2p3/2. Two deconvoluted Ti 2p3/2 peaks were located at approximately 458.1 and 458.7 eV in both catalysts, which correspond to Ti3+ and Ti4+ valences, respectively. The corresponding Ti 2p1/2 peaks were located at approximately 463.6 and 464.5 eV, which were also assigned to Ti3+ and Ti4+ valences. The atomic ratios of Ti4+ to Ti3+ are 0.88 and 2.09 for the pure TiO2 and N–TiO2 (1–8) samples, respectively. The above results suggest that nitrogen doping suppressed the formation of Ti3+ defects. These defects hinder the photocatalytic reactions by increasing the possibility of the recombination of electron–hole pairs.47 As a result, enhanced photocatalytic activity can be expected when N–TiO2 (1–8) is used as a photocatalyst.
As mentioned, to increase the photocatalytic activity of TiO2, it is crucial to enhance the visible light absorption, which can be realized through band structure engineering. Fig. 7 compares the UV-Vis-DRS of the pristine TiO2, the nitrogen-doped TiO2 samples and the commercial TiO2 (P25). The as-prepared pristine TiO2 microspheres displayed a threshold in the UV response at 400 nm, corresponding to an estimated band gap energy (Ebg) of 3.1 eV, whereas the P25 sample had a light absorption edge of 414 nm and an Ebg of 3.0 eV. With an increasing amount of nitrogen, the absorption intensity at wavelengths below 400 nm decreased continuously, indicating reduced UV activity; this is especially true for the N–TiO2 (1–16) sample. In addition, the adsorption edge of the nitrogen-doped samples increased continuously. N–TiO2 (1–4) had a light adsorption edge of 433 nm, corresponding to an Ebg of 2.86 eV. As shown in Fig. 7, the samples with higher nitrogen doping, i.e., N–TiO2 (1–8) and N–TiO2 (1–16), had a similar threshold at 470 nm, with an estimated Ebg of 2.64 eV, which further confirmed the Ns species in the N–TiO2 (1–8) sample, as evidenced by the XPS results. It can be concluded that the N–TiO2 (1–8) sample is a good candidate as a catalyst for the photo-degradation of dyes because of its enhanced visible light response and comparable UV activity to the TiO2 and N–TiO2 (1–4) samples. It was previously reported that nitrogen doping can lead to a mixing of the N 2p orbital with the O 2p orbital to form intermediate energy levels. This action would either shift the absorption edge toward the visible light region or form an isolated narrow N 2p band above the O 2p valence band (VB) to enhance the visible light response as shown in Fig. S5† (band diagram of pristine TiO2 and N–TiO2).39,43 In this case, because there is no distinct peak corresponding to a doping level in the UV-Vis spectra, nitrogen doping from urea likely results in the combination of the N 2p with O 2p orbitals to yield an intermediate energy level. This promotes electronic excitation from the valence band to the intermediate energy level by the absorption of visible light.
 | ||
| Fig. 7 UV-Vis diffuse reflectance spectra of P25, pristine TiO2 and various nitrogen-doped TiO2 samples with different urea to TiO2 ratios. | ||
A model photocatalytic reaction (MO degradation) was selected to test the photocatalytic activity of the as-prepared nitrogen-doped TiO2 microspheres, and for comparison, commercial anatase TiO2 and P25 with mixed anatase and rutile phases were also tested. To exclude the masking effect of surface adsorption on the photocatalytic activity, the test was conducted after thirty minutes to allow for the establishment of the adsorption–desorption equilibrium between MO and the catalysts in the dark. As shown in Fig. 8a, the dye adsorption is typically less than 7%, and the differences in the dye adsorption ratios for the various photocatalysts in the dark are not likely to have a strong effect on the photocatalytic activity.7 Once the light was turned on, different responses to the relative concentration of the dye in water with irradiation time for the various photocatalysts were observed, suggesting their different photocatalytic activities. The data were also plotted in semi logarithmic form to calculate the first-order reaction rate constants (k). As shown in Fig. 8b, the k values were 0.0009, 0.0022, 0.0026, 0.0039, 0.0049 and 0.0126 min−1 for anatase TiO2, TiO2 microspheres calcined at 600 °C, P25, TiO2 microspheres calcined at 400 °C, N–TiO2 (1–16) and N–TiO2 (1–8), respectively. Among the various photocatalysts, the N–TiO2 (1–8) displayed the highest photocatalytic activity by far. As mentioned previously, the photocatalytic activity of a catalyst is determined by many factors such as band gap, specific surface area, particulate morphology and phase compositions.7–10,27–30 According to the SEM observations, the as-prepared pristine TiO2 had a microspherical morphology similar to the structure of N–TiO2 (1–8) (both calcined at 400 °C), with comparable specific surface areas. However, the significantly enhanced catalytic activity of N–TiO2 (1–8) over that of the pristine TiO2 microspheres is clearly related to the nitrogen doping. As demonstrated previously, the nitrogen doping effectively extended the light absorption edge into the visible light region (400 nm to 470 nm), thus increasing photocatalytic efficiency.
 | ||
| Fig. 8 (a) Photocatalytic degradation of MO under UV and visible light irradiation. (b) First-order reaction rate constants (k) vs. reaction time in the presence of different photocatalysts. | ||
According to Fig. 8, the as-prepared pristine anatase TiO2 (calcined at 600 °C) showed much better photocatalytic activity than the commercial anatase TiO2 (0.0022 vs. 0.0009 min−1) and was almost as high as that of P25, with rich anatase–rutile phase junctions (0.0026 min−1). It should be noted that the as-prepared pristine TiO2 calcined at 600 °C, commercial P25 and anatase TiO2 powder samples all had similar BET surface areas of approximately 60 m2 g−1 (Fig. S6†), and the 600 °C calcined TiO2 microspheres were also of a single anatase phase,48 indicating a reasonable basis for comparison of the photocatalytic activities of these three TiO2 materials. As mentioned, the photocatalytic activity of a catalyst is closely related to its particulate morphology, which can affect the recombination of electron–hole pairs; the nanostructure facilitates the photocatalytic reactions of TiO2.7–10 After calcination at 600 °C, the hierarchical porous microspherical structure with some mesopores, which were also observed for the as-prepared TiO2 sample calcined at 400 °C, was still maintained (Fig. S6 and S7a†). On the other hand, the commercial P25 and anatase TiO2 powders only had micropores and nanoparticles with different particle sizes, as shown in Fig. S6b and S7.† Such a special morphological structure of the as-prepared TiO2 microspheres likely improved the interfacial charge transfer and suppressed the recombination of electron–hole pairs. These beneficial effects contribute to the superior photocatalytic activity of the as-prepared TiO2 (600 °C), compared to the commercial TiO2 materials.
The high specific surface area (116 m2 g−1), rich anatase TiO2–TiO2(B) phase junctions, specific microspherical particulate morphology with mesopores and the hierarchical micro/nano structures, and the nitrogen doping resulted in the superior photocatalytic performance of the N–TiO2 (1–8) catalyst. Interestingly, the photocatalytic activity of nitrogen-doped TiO2 was found to decrease drastically upon further increasing the nitrogen content/urea amount, which is similar to the results of nitrogen-doped TiO2 and NaTaO3 in literature.19,49,50 More specifically, after 180 min of irradiation, approximately 75% of the MO was degraded by the N–TiO2 (1–8) photocatalyst, whereas the values were 42%, 36% and 21% for N–TiO2 (1–16), TiO2 microspheres@400 °C and P25, respectively. The possible reason for such a difference between N–TiO2 (1–8) and N–TiO2 (1–16) is that excessive nitrogen doping sites may act as recombination centers for photo-induced electrons and holes, leading to a significant reduction in activity.
The recombination of the charge carriers can be explored via the PL emission spectra of pristine TiO2, N–TiO2 (1–8) and N–TiO2 (1–16) samples excited by 320 nm UV light as shown in Fig. S3.† Higher PL intensity means easier recombination of the photo-generated electrons and holes. The PL spectrum of pristine TiO2 showed the strongest emission from 400 to 550 nm at room temperature, suggesting the rapid recombination of the photo-generated electrons and holes in pristine TiO2 and then low photocatalytic activity. In contrast, the relatively low PL intensity for nitrogen-doped TiO2 indicates that the recombination of photo-induced electrons and holes of TiO2 can be suppressed by nitrogen doping. Moreover, N–TiO2 (1–8) showed lower PL intensity than N–TiO2 (1–16) since excessive nitrogen doping sites acted as recombination centers for photo-induced electrons and holes, which is in good agreement with the results of photocatalytic activity.
The efficiency of reusable photocatalysts is also very important for practical applications.18,50 In this study, we tested the cycling performance of the N–TiO2 (1–8) photocatalyst. No obvious deactivation of the N–TiO2 (1–8) photocatalyst was observed in the cycling tests for three runs, as shown in Fig. S8,† which reveals the outstanding photocatalytic stability of the catalyst. The degradation ratios of MO after 150 min of reaction were 74.4%, 72.0% and 70.3% for the first run, second run and third run, respectively. Fig. S9† is the SEM image of the photocatalyst after three cycles, and it can be seen that the porous microspherical morphology was well maintained, which enabled the high photocatalytic activity and effective suppression in the recombination of photo-generated electron–hole pairs.
Conclusions
In summary, nitrogen-doped porous TiO2 microsphere-based photocatalysts with hierarchical nano and microstructures have been successfully synthesized by a two-step method, which includes a solvothermal process and a urea-based solid state reaction. Yellowish N–TiO2 exhibits rich phase junctions between anatase TiO2 and TiO2(B) crystalline phases, as evidenced by the XRD and TEM results. Nitrogen doping can narrow the band gap of the pristine TiO2 and then enhance the absorption of visible light. The hierarchical structure and abundant phase junctions of the photocatalysts can promote the charge carrier separation, which enhanced the photocatalytic activity remarkably. However, excessive nitrogen doping can destroy the microspherical morphology and generate lattice defects that serve as recombination centers, which are harmful for photocatalytic reactions. As a result, N–TiO2 (1–8) exhibits a higher photocatalytic activity than the benchmark P25, pure anatase phase TiO2 nanoparticles and microspheres and N–TiO2 (1–16). In addition, the N–TiO2 (1–8) photocatalyst displays good cycling performance. We propose that the high photocatalytic activity of N–TiO2 (1–8) arises primarily from the synergistic effects of the hierarchical microstructure, rich phase junctions and the enhanced absorption of visible light. Therefore, the nitrogen-doped TiO2 microspheres can be promising photocatalysts for various photocatalytic applications.Acknowledgements
The authors would like to thank the Australia Research Council for supporting the project under contract FT100100134. Dr Wei Wang also acknowledges Curtin University for a postdoctoral fellowship. The authors acknowledge the facilities, scientific and technical assistance of the Curtin University Electron Microscope Facility and Curtin X-Ray Laboratory, both of which are partially funded by the University, State and Commonwealth Governments.Notes and references
- S. M. A. Shibli, P. S. Arun and A. V. Raj, RSC Adv., 2015, 5, 19393 RSC.
- L. Guo, Z. Yang, B. Zu, B. Lu and X. Dou, RSC Adv., 2016, 6, 358 RSC.
- J. E. Benedetti, D. R. Bernardo, A. Morais, J. Bettini and A. F. Nogueira, RSC Adv., 2015, 5, 33914 RSC.
- Z. Shen, S. Sun, W. Wang, J. Liu, Z. Liu and J. C. Yu, J. Mater. Chem. A, 2015, 3, 3285 CAS.
- Z. Jin, Q. Zhang, S. Yuan and T. Ohno, RSC Adv., 2015, 5, 4026 RSC.
- L. Xin and X. Liu, RSC Adv., 2015, 5, 71547 RSC.
- H. B. Wu, H. H. Hng and X. W. Lou, Adv. Mater., 2012, 24, 2567 CrossRef CAS PubMed.
- J. B. Joo, I. Lee, M. Dahl, G. D. Moon, F. Zaera and Y. Yin, Adv. Funct. Mater., 2013, 23, 4246 CrossRef CAS.
- J. H. Pan, X. Z. Wang, Q. Huang, C. Shen, Z. Y. Koh, Q. Wang, A. Engel and D. W. Bahnemann, Adv. Funct. Mater., 2014, 24, 95 CrossRef CAS.
- H. Liu, J. B. Joo, M. Dahl, L. Fu, Z. Zeng and Y. Yin, Energy Environ. Sci., 2015, 8, 286 CAS.
- W. Wang, M. O. Tadé and Z. P. Shao, Chem. Soc. Rev., 2015, 44, 5371 RSC.
- S. Krejčíková, L. Matějová, K. Kočí, L. Obalová, Z. Matěj, L. Čapek and O. Šolcová, Appl. Catal., B, 2012, 111–112, 119 CrossRef.
- J. G. Yu, Q. J. Xiang and M. H. Zhou, Appl. Catal., B, 2009, 90, 595 CrossRef CAS.
- J. Zhang, C. Pan, P. Fang, J. Wei and R. Xiong, ACS Appl. Mater. Interfaces, 2010, 2, 1173 CAS.
- C. Chen, P. Li, G. Wang, Y. Yu, F. Duan, C. Chen, W. Song, Y. Qin and M. Knez, Angew. Chem., Int. Ed., 2013, 52, 9196 CrossRef CAS PubMed.
- B. Sambandam, A. Surenjan, L. Philip and T. Pradeep, ACS Sustainable Chem. Eng., 2015, 3, 1321 CrossRef CAS.
- C. McManamon, J. O'Connell, P. Delaney, S. Rasappa, J. D. Holmes and M. A. Morris, J. Mol. Catal. A: Chem., 2015, 406, 51 CrossRef CAS.
- T. Wang, X. Yan, S. Zhao, B. Lin, C. Xue, G. Yang, S. Ding, B. Yang, S. Ding, B. Yang, C. Ma, G. Yang and G. Yang, J. Mater. Chem. A, 2014, 2, 15611 CAS.
- A. Tarasov, G. Trusov, A. Minnekhanov, D. Gil, E. Konstantinova, E. Goodilin and Y. Dobrovolsky, J. Mater. Chem. A, 2014, 2, 3102 CAS.
- Z. Xiong and X. S. Zhao, J. Am. Chem. Soc., 2012, 134, 5754 CrossRef CAS PubMed.
- M. Xie, Y. Feng, Y. Luan, X. Fu and L. Jing, ChemPlusChem, 2014, 79, 737 CrossRef CAS.
- X. Chen and C. Burda, J. Am. Chem. Soc., 2008, 130, 5018 CrossRef CAS PubMed.
- T. Lin, C. Yang, Z. Wang, H. Yin, X. Lü, F. Huang, J. Lin, X. Xie and M. Jiang, Energy Environ. Sci., 2014, 7, 967 CAS.
- K. Maeda and K. Domen, J. Phys. Chem. C, 2007, 111, 7851 CAS.
- M. Kitano and M. Hara, J. Mater. Chem., 2010, 20, 627 RSC.
- R. Abe, J. Photochem. Photobiol., C, 2010, 11, 179 CrossRef CAS.
- X. Li, P. Liu, Y. Mao, M. Xing and J. Zhang, Appl. Catal., B, 2015, 164, 352 CrossRef CAS.
- K. Selvam and M. Swaminathan, RSC Adv., 2012, 2, 2848 RSC.
- J. Zhang, Q. Xu, Z. C. Feng, M. J. Li and C. Li, Angew. Chem., Int. Ed., 2008, 47, 1766 CrossRef CAS PubMed.
- D. Yang, H. Liu, Z. Zheng, Y. Yuan, J. Zhao, E. R. Waclawik, X. Ke and H. Zhu, J. Am. Chem. Soc., 2009, 131, 17885 CrossRef CAS PubMed.
- Z. Wang, K. Lv, G. Wang, K. Deng and D. Tang, Appl. Catal., B, 2010, 100, 378 CrossRef CAS.
- M. Bellardita, A. Di Paola, L. Palmisano, F. Parrino, G. Buscarino and R. Amadelli, Appl. Catal., B, 2011, 104, 291 CrossRef CAS.
- J. Wang, Y. Zhou and Z. P. Shao, Electrochim. Acta, 2013, 97, 386 CrossRef CAS.
- L. G. Devi and R. Kavitha, Mater. Chem. Phys., 2014, 143, 1300 CrossRef CAS.
- Y. Niu, M. Xing, J. Zhang and B. Tian, Catal. Today, 2013, 201, 159 CrossRef CAS.
- G. Yang, Z. Jiang, H. Shi, T. Xiao and Z. Yan, J. Mater. Chem., 2010, 20, 5301 RSC.
- V. Kumar, Govind and S. Uma, J. Hazard. Mater., 2011, 189, 502 CrossRef CAS PubMed.
- S. Sakthivel, M. Janczarek and H. Kisch, J. Phys. Chem. B, 2004, 108, 19384 CrossRef CAS.
- R. Asahi and T. Morikawa, Chem. Phys., 2007, 339, 57 CrossRef CAS.
- C. Di Valentin, G. Pacchioni, A. Selloni, S. Livraghi and E. Giamello, J. Phys. Chem. B, 2005, 109, 11414 CrossRef CAS PubMed.
- C. Di Valentin, E. Finazzi, G. Pacchioni, A. Selloni, S. Livraghi, M. C. Paganini and E. Giamello, Chem. Phys., 2007, 339, 44 CrossRef CAS.
- H. U. Lee, Y. Lee, S. C. Lee, S. Y. Park, J. W. Lee, C. Lim, C. Choi, M. Choi, S. Y. Lee, Y. Oh and J. Lee, Chem. Eng. J., 2014, 254, 268 CrossRef CAS.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269 CrossRef CAS PubMed.
- J. Wang, D. N. Tafen, J. P. Lewis, Z. Hong, A. Manivannan, M. Zhi, M. Li and N. Wu, J. Am. Chem. Soc., 2009, 131, 12290 CrossRef CAS PubMed.
- F. Peng, L. Cai, H. Yu, H. Wang and J. Yang, J. Solid State Chem., 2008, 181, 130 CrossRef CAS.
- J. Wang, H. Li, H. Li, S. Yin and T. Sato, Solid State Sci., 2009, 11, 182 CrossRef CAS.
- A. Kasahara, K. Nukumizu, T. Takata, J. N. Kondo, M. Hara, H. Kobayashi and K. Domen, J. Phys. Chem. B, 2003, 107, 791 CrossRef CAS.
- Y. Cai, B. T. Zhao, J. Wang and Z. P. Shao, J. Power Sources, 2014, 253, 80 CrossRef CAS.
- D. Nassoko, Y. Li, H. Wang, J. Li, Y. Li and Y. Yu, J. Alloys Compd., 2012, 540, 228 CrossRef CAS.
- D. Liu, Y. Jiang and G. Gao, Chemosphere, 2011, 83, 1546 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Rietveld analyses of XRD patterns, FTIR spectra, nitrogen adsorption/desorption isotherm patterns, BJH pore size distribution curves, SEM images and multiple cycled performance. See DOI: 10.1039/c6ra02966c |
| This journal is © The Royal Society of Chemistry 2016 |


