Identification of key umami-related compounds in Yangtze Coilia ectenes by combining electronic tongue analysis with sensory evaluation†
Jun Gong‡
a,
Hui Shen‡a,
Jin-yuan Zheng‡a,
Ning-ping Tao*a,
Sai-qi Gub,
Yaowen Huanga and
Mingfu Wanga
aCollege of Food Science and Technology, Shanghai Ocean University, No.999, Hucheng Ring Rd, Lingang New City, Shanghai, 201306, P.R. China. E-mail: nptao@shou.edu.cn; Fax: +86-21-61900365; Tel: +86-21-61900380
bOcean College, Zhejiang University of Technology, No. 18, Chaowang Road, Hangzhou, Zhejiang Province 310014, P. R. China
First published on 4th May 2016
Abstract
The Yangtze Coilia ectenes is a delicious fish known for its distinctive aroma, intense umami taste and high nutritional value. The analysis of Yangtze Coilia ectenes flesh extract using an e-Tongue indicated that saltiness and umami taste were the primary sensory attributes. The relationship between umami-related components and umami taste of this fish was studied by using an electronic tongue (e-Tongue) and sensory perception analysis. As free amino acids, nucleotides and inorganic ions are the main contributors for umami, their contents in Yangtze Coilia ectenes flesh extract were quantified instrumentally and a synthetic sample composed of similar contents of free amino acids, nucleotides and inorganic ions was prepared subsequently. No significant difference in umami taste between that of the synthetic sample and the flesh extract were observed by using the e-Tongue and sensory analysis (p < 0.05). Our further analysis using an omission test indicated nine components including Glu, Gly, AMP, IMP, GMP, K+, Na+, Cl− and PO43− are the key umami-related components with a proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 for amino acids, nucleotides and inorganic ions, respectively. Our results indicated that a simplified synthetic extract can replicate the umami taste of Yangtze Coilia ectenes flesh which provides solid evidence for the preparation of new flavor enhancers in the future.
40 for amino acids, nucleotides and inorganic ions, respectively. Our results indicated that a simplified synthetic extract can replicate the umami taste of Yangtze Coilia ectenes flesh which provides solid evidence for the preparation of new flavor enhancers in the future.
1. Introduction
Yangtze Coilia ectenes is a small-to-medium sized fish.1 The fish is famous for its umami taste, and is known as one of the “three most delicious fish in the Yangtze River” together with two other species (hilsa herring and obscure puffer fish).2 It is a valuable migratory fish found in the middle and lower reaches of the Yangtze River and its affiliated lakes,3,4 migrating from offshore to fresh water areas during the spawning season every spring.5 It is known that the umami taste and tender texture of the fish reach their peak before they swim upstream to spawn.Umami is the fifth basic taste along with sourness, sweetness, bitterness and saltiness.6 Several groups of non-volatile compounds including amino acids, 5′-nucleotides, organic acids and inorganic ions have been suggested to affect the umami taste.7 Among amino acids, aspartic acid (Asp) and glutamic acid (Glu) are called umami amino acids and found to have an effect on the umami taste of fish; glycine (Gly), histidine (His) and alanine (Ala) are also thought to contribute to the umami taste of seafood providing some sweetness or bitterness.8 In addition to free amino acids, the 5′-nucleotides, including 5′-cytosine monophosphate (5′-CMP), 5′-uridine monophosphate (5′-UMP), 5′-adenosine monophosphate (5′-AMP), 5′-inosine monophosphate (5′-IMP), 5′-guanosine monophosphate (5′-GMP) and 5′-xanthosine monophosphate (5′-XMP), also play important roles for the taste of food with 5′-AMP, 5′-IMP, 5′-GMP and 5′-XMP known as umami 5′-nucleotides.9 Organic acids, in particular, succinic acid, have also been found to be umami-enhancing compounds and can increase the umami intensity of MSG.10 Inorganic ions such as K+, Na+, Mg2+, Cl−, PO43− have been shown to significantly affect the sensory quality of fish9 and the omission of these ions can cause sweetness and umami to disappear, and bitterness to increase significantly.11
The umami taste of food is not only made up by the simple accumulation of umami-related components, but also by the synergistic interactions among them. As an example, Yamaguchi et al. investigated the synergy between amino acids and nucleotides, and deduced an empirical formula for the equivalent umami concentration (EUC), which helps to quantify synergistic effects on umami intensity.12 Schiffman et al. demonstrated an interaction between L-glutamate and inorganic cations. The addition of sodium and potassium salts reduced the taste thresholds of Glu, but this effect was not observed when salts were added to MSG solutions.13 To figure out the umami taste of a special food, it is better to evaluate the interaction of different umami components.
Due to the complexity and synergistic interactions among umami substances, quantitative analysis of umami taste should be assessed by sensory evaluation. However, sensory evaluation is susceptible to human physical, psychological conditions and other environmental influences; the electronic tongue (e-Tongue) can be used to complement sensory valuation. As examples, the e-Tongue was used to analyze the umami taste of MSG, IMP and GMP14 and also quantify umami taste in tomatoes in conjunction with a sensory panel.15 The omission tests, known as removing a compound or a group of compounds from a mixture for sensory analysis, are often used to figure out the importance of different components for sensory contribution.10 In the past, some studies have used omission tests to investigate the taste-activity components of some foodstuffs such as black tea,16 tomato juice,17 mature Cheddar cheese10 and oval squid Sepioteuthis lessoniana.18
In the present study, the umami taste in Yangtze Coilia ectenes, a delicious fish was investigated using a combination of instrumental analysis, sensory analysis and e-Tongue analysis to identify the key umami-related components and figure out their interactions.
2. Materials and methods
2.1 Materials and reagents
All the following reagents were of analytical grade and obtained commercially. Monopotassium phosphate (KH2PO4), HPLC-grade methanol (MeOH), L-aspartic acid (Asp), L-glutamic acid (Glu), glycine (Gly), alanine (Ala), histidine (His), amino acid standard, 5′-cytosine monophosphate (5′-CMP), 5′-uridine monophosphate (5′-UMP), 5′-adenosine monophosphate (5′-AMP), 5′-inosine monophosphate (5′-IMP) and 5′-guanosine monophosphate (5′-GMP) were purchased from Sigma-Aldrich Chemical Co. (Shanghai, P. R. China). Sodium hydroxide (NaOH), potassium hydroxide (KOH), phosphoric acid (H3PO4) and hydrochloric acid (HCl) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, P. R. China). The pure water used in the study was obtained using a MilliQ system. Yangtze Coilia ectenes (average body mass 120 g) used in this study were harvested by Jiangsu Zhongyang Group Co., Ltd. (Nantong city, Jiangsu Province, P. R. China) in March 2013, kept on ice and transported to own laboratory within 48 hours.2.2 Sample preparation
After removing the head, skin, tail and viscera the Yangtze Coilia ectenes fleshes were uniformly mixing and then kept at −80 °C. Prior to the experiment, the samples were taken out from the freezer and steamed for 15 min to simulate the daily cooking conditions. To prepare the sample for quantitative analysis, an extract of steamed flesh was prepared according to the method of Hatae et al.19The steamed flesh was firstly mixed with 6% perchloric acid solution at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w
10 (w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) w) and homogenized using an Ultra Turrax (T 18, IKA, Shanghai, P. R. China). The homogenate was then sonicated for 15 min at 40 °C and centrifuged at 11
w) and homogenized using an Ultra Turrax (T 18, IKA, Shanghai, P. R. China). The homogenate was then sonicated for 15 min at 40 °C and centrifuged at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 20 min at 4 °C. The precipitate was re-extracted once more as described above. The combined supernatants were collected and filtered using a Whatman No. 54 filter paper. For the sensory evaluation, the flesh extract was prepared with the same procedures except using cold water to replace 6% perchloric acids for extraction.
000 × g for 20 min at 4 °C. The precipitate was re-extracted once more as described above. The combined supernatants were collected and filtered using a Whatman No. 54 filter paper. For the sensory evaluation, the flesh extract was prepared with the same procedures except using cold water to replace 6% perchloric acids for extraction.
2.3 Analysis of free amino acids
The amino acid composition was determined using an amino acid auto-analyzer (L-8800, Hitachi, Tokyo, Japan) using ninhydrin as the post-column reagent.2.4 Analysis of 5′-nucleotides
The nucleotides were quantified by HPLC with a Welch Ultimate AQ-C18 HPLC column (250 mm × 4.6 mm, 5 μm), using an isocratic elution system. The mobile phase was consisted of 20 mM of KH2PO4 in pure water, with pH adjusted to 4.50 using H3PO4. The total run time was 30 min and the sample injection volume was 10 μL. The flow rate was 1 mL min−1, the column temperature was set at 30 °C, and the UV detection wavelength was set at 254 nm. The nucleotides were identified by the comparison of retention times with individual standards and quantification was done via external calibration curves.2.5 Analysis of inorganic ions
Sodium, potassium, phosphorus and calcium ions were determined using a flame atomic absorption instrument (ZEEnit 700, Analytik Jena, Shanghai, China).20 Chloride was determined using diagnostic kits from BioAssay Systems (Hayward, CA, USA). Phosphate was estimated photometrically after conversion into molybdenum blue.212.6 Equivalent umami concentration (EUC)
EUC (g MSG/100 g) is the concentration of MSG equivalent to the umami intensity given by the mixture of umami amino acids and 5′-nucleotides and the formula is showed below:12| Y = ∑aibi + 1218(∑aibi)(∑ajbj) | (1) |
2.7 The preparation of synthetic extract
Fifteen components (Asp, Glu, Gly, His, Ala, 5′-CMP, 5′-UMP, 5′-AMP, 5′-IMP, 5′-GMP, K+, Na+, Mg2+, Cl− and PO43−) were chosen according to their known effect on umami taste. These substances were dissolved individually in pure water to prepare stock solutions. In accordance with the chemical profile of Yangtze Coilia ectenes, the stock solutions were then added together to obtain the synthetic extract and the pH was adjusted to 6.8 with sulfuric acid.2.8 Sensory analysis
The sensory panel consisted of 21 trained panelists using reference solutions (MSG) for umami attributes training. Their sense of smell was suppressed by using nose clips, and they were instructed to cleanse their mouth with soda bread and pure water between samples to prevent carry-over of umami taste and to avoid sensory fatigue. In addition experiments, samples were presented in a sequence of increasing concentration of components to reduce any carry-over effect, and were spat out after tasting. The synthetic extract, flesh extract and other samples were assessed on a 10 cm unstructured linear scale anchored from “no sensation” (value 0) to “strong sensation” (value 10) with a mark on the middle (value 5) corresponding to 1.08 g MSG solution.212.9 Omission test
An omission test was used to evaluate the relative impact of each substance on the umami taste. All the components in the synthetic extract were divided into three groups: group A, amino acids; group B, flavor nucleotides and group C, inorganic ions. Partial synthetic extracts lacking in a group or a single compound were also prepared. Panelists were asked to assess the differences using the triangle test, where the subject must identify one stimulus out of three that is different from the other two.22 The panelists who selected the correct sample were asked whether the umami intensity had decreased. For the simplified synthetic extract the assessors were asked to differentiate between the synthetic extract and the simplified synthetic extract using the triangle test.2.10 Addition test
A range (20%, 40%, 60%, 80% and 100%) of concentrations of each compound identified in Yangtze Coilia ectenes flesh, were added respectively to the simplified synthetic extract. All the samples were evaluated by the panelists on a 16 cm unstructured linear scale anchored from “suppress” (value −8) to “contribute” (value +8).2.11 e-Tongue analysis
The α-Astree e-Tongue (Alpha M.O.S. Co., Toulouse, France) consists of a sensor array made up of seven different liquid cross-selective sensors (SRS, GPS, STS, UMS, SPS, SWS and BRS) and an Ag/AgCl reference electrode. It also contains a 16-position auto-sampler, an electronic unit for data acquisition and a personal computer with a complete software package for auto-sampler control and pattern recognition methods. Before e-Tongue analysis, the sensor coatings had to pass at least two out of three test steps (conditioning, calibration and diagnostic) in order to reach a constant potential.The e-Tongue was rinsed with de-ionized water in between measurements. All samples were analyzed in six replicates (three for conditioning, three for detecting) at room temperature, and each analysis lasted for 120 s. To determine the taste profile of Yangtze Coilia ectenes flesh, 80 mL of the flesh extract, the synthetic extract, 10 mM citric acid, 12 mM sodium chloride (NaCl), 4 mM monosodium glutamate (MSG), 40 mM sucrose and 8 mM quinine were analyzed using the e-Tongue. Then the flesh extract, simplified synthetic extract and simplified synthetic extract with key components individually removed were analyzed to obtain the response values of each sample to umami intensity.
2.12 Statistical analysis
All quantitative analysis was performed in triplicate. The results of chemical analyses and score evaluations were recorded as mean ± standard deviation. Analysis of variance (ANOVA) and Duncan tests were processed using SPSS (Version 17.0, SPSS Inc., Chicago, USA). The results of the triangle tests were statistically processed using a table of criteria. For the e-Tongue data, principal component analysis (PCA), a multivariate analysis, was used for extracting useful information and eliminating the overlapped information by data reconstruction and dimensional reduction. At the same time, the specific sensor array module of the software Alpha Soft Version 12.3 which came with the Astree system was used to analyze the raw data. Histogram ordinate values (the measurements) were used to indicate the relative intensity of the umami taste, that is, the instrument response value of umami taste.3. Results and discussion
3.1 Determination of the taste profile of Yangtze Coilia ectenes flesh using the e-Tongue
Multivariate data analysis was used to process the data collected by the e-Tongue, and the determination of grouping similarity and groupings of observations were performed using PCA.23 The PC1, PC2 and PC3 axes show the contribution rates of the first three principal components of the PCA obtained in transition. The higher the contribution rate, the more the aggregative indicator of dimensionality reduction reflects the information of the original indicator. The scatter plot of the PCA shows the significant 2-dimension scatter plot and 3-dimension scatter plot which can be obtained by plotting PC1 against PC2 or PC3. Fig. 1 shows the PCA map of the flesh extract data. Reference solutions of citric acid, sodium chloride, sucrose, quinine and monosodium glutamate are used to represent the five basic tastes. As shown in Fig. 1, the accumulative contribution rate of the former two-dimensional principal component accounted for 97.5%, the accumulative contribution rate of the former three-dimensional principal component accounted for 99.2%, indicating that the data can represent most of the information of original data. The smaller the euclidean distance of two samples in PCA map, the more similar their tastes were.24 The closest in euclidean distance of the five basic tastes from the flesh samples were NaCl and MSG, which means that umami and saltiness are the predominant tastes. | ||
| Fig. 1 Two principal components analysis (PCA) for the natural extract, synthetic extract, citric acid, sodium chloride (NaCl), sucrose, quinine and monosodium glutamate (MSG). | ||
3.2 Umami comparison between the flesh extract and the synthetic extract
As free amino acids, nucleotides and inorganic ions are the main contributors for umami, their contents in Yangtze Coilia ectenes flesh extract were quantitated instrumentally and a synthetic sample composed of similar contents of free amino acids, nucleotides and inorganic ions was prepared according to the analytical data shown in Table 1. Then the flesh extract and synthetic extract was applied to sensory evaluation and e-Tongue analysis. As showed in Fig. 2, no significant difference was found in the umami tastes between these two solutions (p > 0.05). This demonstrates that the synthetic extract can be considered as a good reproduction of the umami taste of the flesh extract of Yangtze Coilia ectenes, which is consistent with the e-Tongue results (Fig. 1). However, the scores of both the synthetic extract and the flesh extract were significantly higher than the EUC of Yangtze Coilia ectenes. This implies that the EUC does not completely represent the umami taste in a complex matrix (p < 0.05). This may be because the EUC only represents the synergistic effect between amino acids (Glu and Gly) and 5′-nucleotides (GMP, IMP, AMP and XMP), indicating that the presence of other compounds might contribute to the umami taste.| a n.d. = not detected, values are mean ± standard deviation. | |
|---|---|
| Amino acids | |
| Aspartic acid | 1.34 ± 0.04 |
| Asparagine | 1.36 ± 0.07 |
| Threonine | 3.93 ± 0.03 |
| Serine | 4.92 ± 0.14 |
| Glutamic acid | 7.85 ± 0.07 |
| Glutamine | 0.74 ± 0.09 |
| Glycine | 8.35 ± 0.02 |
| Alanine | 18.66 ± 0.07 |
| Valine | 4.93 ± 0.09 |
| Methionine | 2.08 ± 0.09 |
| Isoleucine | 2.86 ± 0.09 |
| Leucine | 5.36 ± 0.04 |
| Tyrosine | 2.01 ± 0.05 |
| Phenylalanine | 1.71 ± 0.09 |
| Lysine | 5.57 ± 0.04 |
| Histidine | 10.76 ± 0.42 |
| Anserine | 3.16 ± 0.31 |
| Arginine | 1.76 ± 0.00 |
| Proline | 5.21 ± 0.19 |
| Taurine | 189.08 ± 1.57 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Nucleotides | |
| CMP | 2.00 ± 0.24 |
| UMP | 0.65 ± 0.01 |
| GMP | 6.63 ± 0.16 |
| IMP | 69.32 ± 2.88 |
| AMP | 4.81 ± 0.11 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Inorganic ions | |
| K+ | 279.5 ± 2.7 |
| Na+ | 69.9 ± 10.1 |
| Ca2+ | n.d. |
| Mg2+ | 24.4 ± 0.9 |
| Cl− | 57.13 ± 2.76 |
| PO43− | 212.8 ± 9.6 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| pH | 6.8 |
3.3 Omission experiment with sensory analysis
The experiments were performed using the synthetic extract, and focused on the umami taste. As showed in Table 2, when group A or B was omitted, the umami taste for the synthetic extract was noted as significantly weaker (p < 0.01). This is in agreement with previous studies reporting the importance of both amino acids and nucleotides for umami attributes.25 Removal of nucleotides caused a noticeable change in the intensity of the umami taste, demonstrating that they play a greater role than amino acids for the umami taste of Yangtze Coilia ectenes, possibly due to their much higher concentration. The synthetic extracts lacking of group C components mainly tasted sour, suggesting that inorganic ions also contribute strongly to the umami taste. Then each member of groups A, B and C was omitted in turn to identify the contribution of each of the taste components to the umami taste perception in Yangtze Coilia ectenes flesh.| Omitted group | No. of correct identifications (n = 21) | Level of significance | Decrease in umami taste |
|---|---|---|---|
| a *p < 0.05, **p < 0.01, ***p < 0.001. | |||
| Group A (amino acids of high quantity or strong taste: Asp, Glu, Gly, Ala, His) | 15 | *** | ** |
| Group B (nucleotides and bases: 5′-CMP, 5′-UMP, 5′-GMP, 5′-IMP, 5′-AMP) | 19 | *** | *** |
| Group C (inorganic ions: K+, Na+, Mg2+, Cl−, PO43−) | 20 | *** | *** |
As shown in Table 3, the removal of Gly (p < 0.05) and Glu (p < 0.01) resulted in a decrease in umami intensity. Therefore, Glu and Gly are the key umami amino acids in Yangtze Coilia ectenes. Our data are consistent with literature. As an example, Gly was identified as a taste-active component that imparted sweetness and a weak umami taste in G. borealis muscle.26 Glu and Asp are both recognized as umami compounds in many types of seafood.27 However, in our study, Asp was found to contribute little to the umami taste of Yangtze Coilia ectenes possibly due to its very low concentration (1.34 mg/100 g). Although Ala was the free amino acid with the highest concentration in Yangtze Coilia ectenes (18.66 mg/100 g), the non-significant difference in taste as a whole was observed after its removal, and the umami taste was not reduced.
| Against synthetic extract | No. of correct identifications (n = 21) | Level of significance | Decline of umami taste |
|---|---|---|---|
| a *p < 0.05, **p < 0.01, ***p < 0.001, — not significant. | |||
| Simplified synthetic extract | 11 | — | — |
Based on the sensory evaluation, 5′-GMP, 5′-IMP and 5′-AMP were judged to be the key umami nucleotides. Reducing 5′-IMP in particular led to a large reduction in umami taste (p < 0.001, Table 3), suggesting that 5′-IMP is the most important nucleotide for umami taste. The omission of 5′-CMP or 5′-UMP was not judged to have a significant effect on umami taste. This might due to their very low concentration in Yangtze Coilia ectenes (2 mg/100 g and 0.65 mg/100 g, respectively).
A significant reduction in the intensity of the umami taste was observed with the omission of K+, Na+, Cl− and PO43− from the synthetic extract suggesting they are also possible contributors to the umami taste for Yangtze Coilia ectenes flesh. Yoko Kani reported K+, Na+ and Cl− were taste-active compounds in the mantle muscle of the oval squid Sepioteuthis lessoniana.18 Research carried out by Hayashi et al. also found that inorganic ions Na+ and Cl− had a significant impact on the present of umami taste in boiled snow crab flesh, and the umami taste completely disappeared when Na+ and Cl− were removed.11 In addition, Kawai showed that Na+ and Cl− had a significant impact on the umami taste and palatability of snow crabs, scallops and clams by carrying out omission tests.9 Thus it can be tentatively concluded that K+, Na+ and Cl− can help to enhance the umami taste for Yangtze Coilia ectenes flesh, although they have no umami attributes themselves. There are very few literature reports concerning the role of PO43− in umami taste. Roldán reported that PO43− can improve sensory features of cooked lamb loins without making any contribution to taste.28 The PO43− ion might contribute to the umami taste of Yangtze Coilia ectenes due to its much higher levels in this fish (212.8 mg/100 g).
As the results of the omission tests show that the umami taste in Yangtze Coilia ectenes is mainly attributed to 9 components: Glu, Gly, 5′-AMP, 5′-IMP, 5′-GMP, K+, Na+, Cl− and PO43−, we further prepared simplified synthetic extracts consisting of the above 9 components, and compared them to the synthetic extract using the triangle test. The results in Table 4 show that these two extracts could not be distinguished each other, and the umami taste of the simplified synthetic extract did not decrease significantly compared to the synthetic extract (p > 0.05).
| Omitted component | No. of correct identifications (n = 21) | Level of significance | Decline of umami taste |
|---|---|---|---|
| a *p < 0.05, **p < 0.01, ***p < 0.001, — not significant. | |||
| Asp | 8 | — | — |
| Glu | 14 | ** | ** |
| Gly | 13 | ** | * |
| His | 11 | — | — |
| Ala | 10 | — | — |
| CMP | 9 | — | — |
| UMP | 11 | — | — |
| GMP | 12 | * | * |
| IMP | 18 | *** | *** |
| AMP | 15 | *** | * |
| K+ | 18 | *** | *** |
| Na+ | 20 | *** | *** |
| Mg2+ | 8 | — | — |
| Cl− | 20 | *** | *** |
| PO43− | 13 | ** | * |
3.4 e-Tongue analysis of flesh extract, simplified synthetic extract and simplified synthetic extract with each key component individually removed for umami comparison
The omission experiments were simultaneously analyzed using the e-Tongue. The response values of the umami taste of the flesh extract, simplified synthetic extract and simplified synthetic extract with each key component individually removed were analyzed through the specific sensor array module (UMS). As shown in Fig. 3, the score of the umami taste of the flesh extract was the highest (7.77), followed closely by the simplified synthetic extract (7.20), showing that the simplified synthetic extract can successfully simulate the umami taste of Yangtze Coilia ectenes. This result was consistent with the results from the sensory analysis. Removal of the key umami-related components caused a decrease in the e-Tongue umami score during the omission experiments, with the exception of Cl−. The e-Tongue umami scores were 5.80 and 6.97 after the removal of Glu and Gly, respectively. There was a bigger decrease in the umami intensity on removal of Glu than Gly, this is probably due to the taste properties of the amino acids. The umami score was 6.31 when PO43− was omitted, suggesting that it makes some contributions to the umami taste. The e-Tongue score of the simplified synthetic extract decreased dramatically when GMP, K+ and Na+ were omitted in turn, suggesting that they play major roles in the umami taste of Yangtze Coilia ectenes. However, the umami score did not significantly change when Cl− was omitted. This might be related to the different perception mechanisms between the e-Tongue and the human tongue. Cl− itself does not have a characteristic umami taste, but works as an assistant flavor group when surrounded by positive ions. The human experiments showed that removal of Cl− made the samples tasteless, but the e-Tongue assesses the umami taste by measuring the strength of the electrical signal produced by the specific response of a biological membrane to several components of umami taste. As it is an assistant flavor group, Cl− was not judged to be a taste compound by the e-Tongue, hence the umami score did not change on removal of Cl−. A similar situation happened with IMP, which has been shown to be one of the most important components of umami taste in Yangtze Coilia ectenes. The umami taste detected by the e-Tongue decreased slightly when IMP was omitted, but the score was still 6.68. Although Yang Yang et al. demonstrated that the e-Tongue was a useful tool to evaluate the umami taste of IMP, GMP and MSG, we found that significant synergistic effects in a complex matrix affected e-Tongue's comprehensive analysis of the umami taste of all of the components in Yangtze Coilia ectenes.14 | ||
| Fig. 3 The umami taste of the simplified synthetic extract, meat extract and omission of each compound using the e-Tongue. | ||
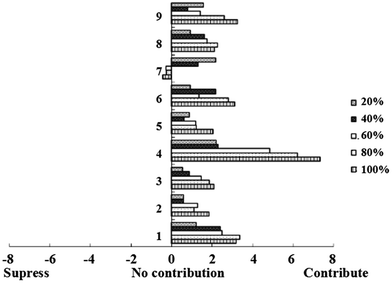
3.5 Addition test to further evaluate key umami components in Yangtze Coilia ectenes
We further investigated the contribution of each key umami-related substance on the umami taste of Yangtze Coilia ectenes by increasing the concentration of each substance in the simplified synthetic extract. As shown in the Fig. 4, all the concentrations of substances showed a positive correlation with umami taste except PO43− (r = −0.913). There are no reports concerning the contribution of PO43− to umami taste, but its high concentration might be one of the reasons why the taste of Yangtze Coilia ectenes changed when it was removed. The specific reasons why the umami taste was suppressed when the concentration of PO43− increased needs to be researched further. GMP (r = 0.989) and IMP (r = 0.974) had a higher correlation coefficients than other umami-related compounds. The results correlate well with the omission experiment, which showed that IMP and GMP are very important components of the umami taste in Yangtze Coilia ectenes. The coefficient of umami taste of GMP (2.3) in Yamaguchi's EUC formula was highest even though its concentration in Yangtze Coilia ectenes was lower than the threshold value. This suggests that GMP exhibits a significant synergistic effect with amino acids. The correlation coefficients of Glu and Gly were both 0.914. Glu is the amino acid with most umami taste, but Gly produces a sweet taste. Yoko Kani found that the umami taste decreased when Gly was eliminated, but the reasons why have not been thoroughly researched.184. Conclusions
In conclusion, through instrumental analysis, e-Tongue analysis, sensory analysis, omission test and addition test, we are able to figure out the key umami components and their ratio for umami taste of delicious Yangtze Coilia ectenes flesh with Glu, Gly, 5′-AMP, 5′-IMP, 5′-GMP, K+, Na+, Cl− and PO43−, identified as the main contributors.References
- K. V. Radhakrishnan, Y. Li, K. V. Jayalakshmy, M. Liu, B. R. Murphy and S. Xie, Fish. Res., 2012, 125, 156–160 CrossRef.
- W. Wu, N. P. Tao and S. Q. Gu, Eur. Food Res. Technol., 2014, 238(2), 237–245 CrossRef CAS.
- Y. Li, S. Xie, Z. Li, W. Gong and W. He, Fish. Sci., 2007, 73, 1224–1230 CAS.
- C. Ma, Q. Cheng and Q. Zhang, Environ. Biol. Fishes, 2011, 91, 243–249 CrossRef.
- C. A. o. F. S. a. S. F. R. I, East China Sea Fisheries Research Institute, 1990, 93–115.
- S. S. Fuke, Trends Food Sci. Technol., 1993, 4(8), 246–251 CrossRef CAS.
- S. Fuke, in Seafoods: chemistry, processing technology and quality, 1994, pp. 115–139 Search PubMed.
- P. Liu, H.-M. Li and Y.-J. Tang, Food Chem., 2012, 132, 1413–1419 CrossRef CAS.
- M. Kawai, H. Uneyama and H. Miyano, J. Health Sci., 2009, 55, 667–673 CrossRef CAS.
- L. T. Andersen, Y. Ardo and W. L. P. Bredie, Int. Dairy J., 2010, 20, 528–536 CrossRef CAS.
- T. Hayashi, K. Yamaguchi and S. Konosu, J. Food Sci., 1981, 46(2), 479–483 CrossRef CAS.
- S. Yamaguchi and A. Kimizuka, Glutamic acid: advances in biochemistry and physiology, 1979, p. 35 Search PubMed.
- S. S. Schiffman, A. E. Frey, J. A. Luboski, M. A. Foster and R. P. Erickson, Physiol. Behav., 1991, 49, 843–854 CrossRef CAS PubMed.
- Y. Yang, Q. Chen, C. Shen, S. Zhang, Z. Gan, R. Hu, J. Zhao and Y. Ni, J. Food Eng., 2013, 116, 627–632 CrossRef CAS.
- K. Beullens, P. Meszaros, S. Vermeir, D. Kirsanov, A. Legin, S. Buysens, N. Cap, B. M. Nicolai and J. Larnmertyn, Sens. Actuators, B, 2008, 131, 10–17 CrossRef CAS.
- S. Scharbert, N. Holzmann and T. Hofmann, J. Agric. Food Chem., 2004, 52, 3498–3508 CrossRef CAS PubMed.
- C. Salles, S. Nicklaus and C. Septier, Food Chem., 2003, 81, 395–402 CrossRef CAS.
- Y. Kani, N. Yoshikawa, S. Okada and H. Abe, Food Res. Int., 2008, 41, 371–379 CrossRef CAS.
- K. Hatae, M. Kagawa, M. Matsumoto, J. Shimada and H. Yamanaka, Bull. Jpn. Soc. Sci. Fish., 1995, 61(4), 619–626 CrossRef CAS.
- Determination of total phosphorus content (spectrometric method), IDF standard 42A, International Dairy Federation, Brussels, Belgium, 1987 Search PubMed.
- J.-F. Yin, Y.-N. Zhang, Q.-Z. Du, J.-X. Chen, H.-B. Yuan and Y.-Q. Xu, Food Res. Int., 2014, 62, 941–946 CrossRef CAS.
- B. Rousseau and M. O'Mahony, Food Quality and Preference, 2000, 11, 457–464 CrossRef.
- M.-X. Zhang, X.-C. Wang, Y. Liu, X.-L. Xu and G.-H. Zhou, Food Chem., 2012, 135, 1463–1470 CrossRef CAS PubMed.
- E. Tokuyama, C. Matsunaga, K. Yoshida, J.-C. Mifsud, T. Irie, M. Yoshida and T. Uchida, Chem. Pharm. Bull., 2009, 57, 382–387 CrossRef CAS PubMed.
- S. Kaneko, K. Kumazawa, H. Masuda, A. Henze and T. Hofmann, J. Agric. Food Chem., 2006, 54, 2688–2694 CrossRef CAS PubMed.
- Y. Kani, N. Yoshikawa, S. Okada and H. Abe, Fish. Sci., 2007, 73, 940–949 CrossRef CAS.
- S. Yamaguchi, T. Yoshikawa, S. Ikeda and T. Ninomiya, J. Food Sci., 1971, 36(6), 846–849 CrossRef CAS.
- M. Roldán, T. Antequera, T. Perez-Palacios and J. Ruiz, Meat Sci., 2014, 97, 69–75 CrossRef PubMed.
Footnotes |
| † Fund: Shanghai Municipal Natural Science Foundation (14ZR1420100). |
| ‡ Jun Gong, Hui Shen and Jin-yuan Zheng contributed equally to this work and are considered co-first authors. |
| This journal is © The Royal Society of Chemistry 2016 |