Facile synthesis of thermo-responsive episulfide group-containing diblock copolymers as robust protecting ligands of gold nanoparticles for catalytic applications†
Dongmei Wanga,
Bingxin Liub,
Jianhua Lüa and
Changli Lü*a
aKey Laboratory of Nanobiosensing and Nanobioanalysis at Universities of Jilin Province, College of Chemistry, Northeast Normal University, Changchun 130024, P. R. China. E-mail: lucl055@nenu.edu.cn; Fax: +86 431 85098768; Tel: +86 431 85099236
bSchool of Mechanical Engineering, Qinghai University, Xining 810016, P. R. China
First published on 5th April 2016
Abstract
We herein report the synthesis of well-defined novel thermo-responsive diblock copolymers, poly(N-isopropylacrylamide-co-2,3-epithiopropyl methacrylate)-block-poly(poly(ethylene glycol) methyl ether methacrylate) P(NIPAM-co-ETMA)-b-P(PEGMA), bearing an episulfide moiety as a ligand via reversible addition fragmentation chain transfer (RAFT) polymerization, followed by assembly into micelles in aqueous solution. The diblock copolymer-stabilized gold nanoparticles (Au NPs@P) could be further obtained by a Au3+-induced episulfide opening reaction and in situ reduction of HAuCl4. The block copolymer played a great role in the stabilization of Au NPs. These Au NPs@P were employed as an efficient catalyst and presented excellent catalytic activity to reduce 4-nitrophenol to 4-aminophenol. The content of PETMA segments, different ratios of Au atoms to sulfur atoms (MRs) of Au NPs@P1 and the dosage of catalytic carriers and reductants had varying degrees of influence on the catalytic activity. It was found that the catalytic activity of Au NPs@P increased with the decreasing content of PETMA segments. The size of the diblock copolymer-stabilized Au NPs also increased and the catalytic activity became higher with the increase of the MRs. The Au NPs@block copolymer with PNIPAM blocks also exhibited excellent temperature-responsive behavior for the catalytic reduction of 4-nitrophenol.
Introduction
The development of metallic nanostructures has been an in-depth inquiry over several decades for its basic scientific interest and the importance of technology in various applications.1–4 Noble metal nanoparticles (NPs) made of gold, platinum, etc., due to their valuable optical and catalytic properties, have been widely used in catalysis, optoelectronic devices and biomedicine.5–11 Especially, gold nanoparticles (Au NPs) have attracted a lot of attention due to their unique properties, which may markedly differ from those of the respective bulk metal.12 Au NPs are the most stable metal nanoparticles and they exhibit unique properties, such as size-related electronic, magnetic, and optical properties. These unique properties give Au NPs a wide range of applications in the fields of catalysis, biological medicine, and new materials exploration.1,3,13,14 In these areas, one of the main problems of nanoparticles is their limited colloidal stability, which will lead to the tendency to form aggregates in aqueous media due to their highly active surface atoms and subsequently will lead to reduced catalytic activity. In order to explore and realize the applications of Au NPs in biomedicine and catalysis, great efforts have been made to improve the colloidal stability of Au NPs. The formation of metal nanoparticles is usually carried out by reduction of metal ions in the presence of a stabilizer like polymers.3 Previous studies showed that the colloidal stability of Au NPs could be significantly improved through polymer coverage of the nanoparticles. The use of different polymers as stabilizers for synthesizing noble metal nanoparticles has gained wide attention, including polymer dendrimers,15 amphiphilic polymers,16 polymeric micelles17 and double-hydrophilic polymers.18 Block copolymers, which have attracted significant interest within the research community due to their self-assembly ability, are considered as ideal soft templates for preparing metal nanoparticles with a special size and dispersion due to their special core–shell structure.19 There are different ways to connect the block polymer to the surface of nanoparticles. The first method is the grafting-to method. The traditional route is to synthesize nanoparticles and polymers individually, then connect them subsequently.13,16 However, due to the steric hindrance on the nanoparticle surface, the grafting density of polymers is relatively low and sometimes it will be limited in further applications.20 The second classical method is the in situ reduction technique.17,21,22 This strategy is simple and convenient.The preparation of Au NPs in polymeric micelles in an aqueous medium has been reported by the direct self-assembly approach.23–25 The majority of block copolymer self-assembly methods depend on the application of amphiphilic block copolymers, including polystyrene-block-poly(D,L-lactide) (PS-b-PLA),4 polystyrene-b-poly(acrylic acid) (PS-b-PAA),26 poly boc-L-tryptophan acryloyloxyethyl ester-b-poly(ethylene glycol) monomethyl ether acrylate [P(L-Trp-HEA)-b-P(PEGMA)],27 poly(2-(methacryloyloxy)ethyl phosphorylcholine)-b-poly(N-isopropylacrylamide-co-2-(N,N-dimethylamino)ethyl methacrylate) (PMPC-b-P(NIPAM-co-DMA)),28 polystyrene-b-polyethylene oxide (PS-b-PEO)29 and so on. Another method is to directly use the double-hydrophilic block copolymer (DHBC) and metal compounds in aqueous solutions to form hybrid micelles. For example, the use of poly(ethylene oxide)-b-poly(acrylic acid) (PEO-b-PAA),18 poly(2-vinylpyridine)-poly(ethylene oxide) (P2VP-b-PEO),23 di-alkylated polyethylenimines (PEI-1R),30 and polyethyleneoxide-polyethyleneimine (PEO)n-b-PEI31 has been reported. The block copolymers with the above examples can improve the stability of Au NPs. Among these polymers, P(PEGMA) or PEO-based block copolymers as a stabilizer are the most attractive representatives because they can endow Au NPs with good water solubility and high colloidal stability. Taking into account biological applications and the protection of the environment, water is by far the most environmentally friendly medium, and it is essential to develop polymer templates in water medium to synthesize and stabilize nanoparticles.
Stimuli-responsive polymers, also known as smart polymers, have attracted considerable attention as they can exhibit discontinuous changes in physical properties upon receiving environmental stimuli such as solvent exchange, temperature, pH, light, a magnetic or electric field, ion factors, chemical or biological molecules, and mechanical stress. These responses are expressed as the nature of the material.32 Among the stimuli-responsive polymers, poly(N-isopropylacrylamide) (PNIPAM) is the most popularly studied thermo-responsive polymer, which can undergo a reversible coil-to-globule phase transition in water around its lower critical solution temperature (LCST) due to a fine hydrophobic–hydrophilic balance in its structure.33,34 Wu et al. found that the hydrophobic and hydrophilic interaction will have different degrees of impact on the LCST in the block copolymer system.35 Smart materials can be found in several application areas such as active delivery in the biomedical sciences, sensors, catalysis and nanoelectronics.17,36 Over the last decade, thermo-responsive block copolymer-stabilized Au NPs have received much attention,37 for example, they can be used as a catalyst in thermo-responsive catalytic systems and the catalytic activity can be regulated by the temperature.38,39
Block copolymers containing atoms or groups capable of coordinating with gold can be attached to the surface of gold nanoparticles to improve the stabilization of the nanoparticles. A lot of electron-rich atoms or groups including carboxylate,18 thiol,40 sulfur,28 nitrogen41 and amino42 can coordinate with gold. In these groups, thiol is widely employed as an anchoring group via a Au-to-thiol bond. However, in theoretical analysis it is very easy to form two sulfur bonds which may enhance the crosslinking of the polymers. It is well known that episulfide compounds have interesting characteristics for chelating heavy metal ions via a ring-opening reaction under acidic or basic conditions.43 The episulfide monomer of 2,3-epithiopropyl methacrylate (ETMA) as a ligand is very stable and has a strong coordination ability for metal. In previous studies the copolymers of ETMA and vinyl monomers have been synthesized and used as reversible reducing and chelating agents and ion exchange membranes.44–46 Until now, no work has been reported for using the new techniques in the polymerization of ETMA.
Herein, we firstly reported a new type of episulfide group-containing thermo-responsive block copolymer, which can be used as effective ligands for the synthesis of Au NPs with enhanced catalytic activity for the reduction of 4-nitrophenol (4-NP). As shown in Scheme 1, the novel block copolymer ligands composed of a biocompatible PEGMA block and a thermo-responsive block consisting of a random copolymer of NIPAM and episulfide monomer of ETMA were synthesized via reversible addition fragmentation chain transfer (RAFT) polymerization. RAFT polymerization was selected due to the fact that this controlled living radical polymerization technique can be performed under mild reaction conditions, and has a high tolerance towards functional groups.47 We synthesized three block copolymers of P(NIPAM-co-ETMA)-b-P(PEGMA) with different contents of PETMA segments, P(NIPAM53-co-ETMA10)63-b-P(PEGMA)111 (P1), P(NIPAM48-co-ETMA27)75-b-P(PEGMA)113 (P2) and P(NIPAM42-co-ETMA35)77-b-P(PEGMA)114 (P3), respectively. The resulting block copolymers can be dispersed in water and self-assembled to form polymer micelles because the block polymer contains hydrophobic PETMA segments, and a stable micelle system is formed because the hydrophilic block is larger than the hydrophobic block. The PETMA segments in P(NIPAM-co-ETMA)-b-P(PEGMA) interact with the Au precursor, whereas the PPEGMA block stabilizes the Au NPs in aqueous solution. As shown in Scheme 2, the diblock copolymer-stabilized Au NPs were obtained through the in situ reduction of gold precursors with NaBH4 in polymer micelle solution because strong acidic HAuCl4 can induce episulfide group ring-opening to form a complex of Au3+–S–P(NIPAM-co-ETMA)-b-P(PEGMA). Through the study we found that the catalytic activity of the diblock copolymer-stabilized Au NPs for 4-NP was dependent on the content of PETMA segments due to the limitation of the reaction diffusion. Especially, the size of the Au NPs could be controlled by adjusting the molar feed ratios (MRs, the ratios of Au atoms to sulfur atoms in the copolymers). Another advantage of our design is that the Au hybrid particles can be stabilized by the water-soluble PEGMA block as the shell, ensuring that the current system has excellent colloidal stability in aqueous solution under the reaction conditions. The resulting Au NPs obtained using P(NIPAM-co-ETMA)-b-P(PEGMA) as a stabilizer had a superior colloidal stability and their potential as catalysts for the reduction of 4-NP in aqueous solutions was investigated. Furthermore, the temperature-dependent catalytic behavior for the reduction of 4-NP based on the Au NPs@block polymers with PNIPAM chains as catalysts was also studied in this work.
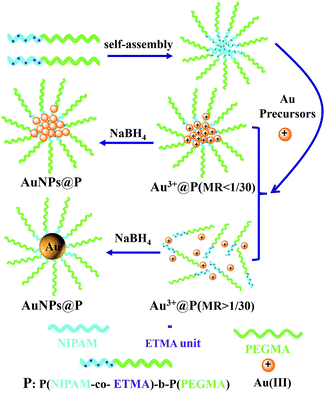 | ||
| Scheme 2 Schematic illustration of the self-assembled structures of block copolymers and Au NPs@block copolymers formed in situ in aqueous solution. | ||
Experimental
Materials
N-Isopropylacrylamide (NIPAM, Aldrich) was recrystallized in hexane. Sodium citrate tribasic dihydrate (99.5%, Mn = 294 g mol−1) and poly(ethylene glycol) methyl ether methacrylate (PEGMA) (99.5%, Mn = 500 g mol−1) were obtained from Sigma-Aldrich and passed through an inhibitor-removing column with aluminum oxide before use. 2,2′-Azobis(2-methylpropionitrile) (AIBN) (98%, Aldrich) was recrystallized twice from ethanol and stored in the dark at 4 °C. The chain transfer agent (CTA), 3-(benzylsulfanylthiocarbonylsulfanyl)-propionic acid (BSPA), was prepared according to previous literature.48 2,3-Epithiopropyl methacrylate (ETMA) was synthesized from GMA based on the method described by Nonaka.49 Gold(III) chloride trihydrate (HAuCl4·3H2O, 99%) and 4-nitrophenol (4-NP, 99%) were obtained from Sinopharm Chemical Reagent Co. Ltd. Tetrahydrofuran (THF) was purified by refluxing over sodium wire and distilled prior to use. All other reagents were analytical grade chemicals and used as received without further purification.Synthesis of the macromolecular chain transfer agent (macro-CTA, P(NIPAM-co-ETMA)-CTA)
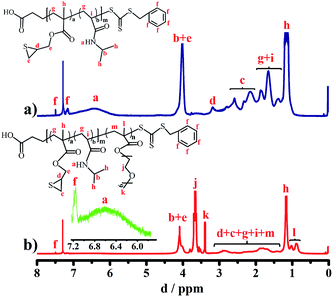
A typical synthesis of P(NIPAM0.84-co-ETMA0.16)63 (A1-CTA) by RAFT polymerization was performed in THF (15 mL) comprising ETMA (0.316 g, 2 mmol), AIBN (0.014 g, 0.086 mmol), NIPAM (4.294 g, 57 mmol) and BSPA (0.233 g, 0.86 mmol). After the solution was degassed using three freeze–pump–thaw cycles, the reaction was performed for 15 h at 70 °C in nitrogen atmosphere under magnetic stirring. The resulting polymer A1-CTA was precipitated three times in petroleum ether before drying in a vacuum oven overnight at room temperature (3.46 g, yield: 75%). In addition, the other macro-CTAs with different proportions of ETMA units, P(NIPAM0.64-co-ETMA0.36)75 (A2-CTA) and P(NIPAM0.55-co-ETMA0.45)77 (A3-CTA), were synthesized by similar route to the one above, and the detailed process and characterization are shown in Table 1. 1H NMR (500 MHz, CDCl3, δ): 7.15–7.48 (f, 5H, Ph), 6.4 (a, 1H, CO–NH–CH(CH3)2),50 4.0 (b, 1H, CO–N–CH(CH3)2),51 3.89–4.27 (e, 2H, CH–CH2),52 3.18 (d, 1H, SCH–), 2.32–2.58 (c, 2H, SCH–CH2), 1.39–1.85 (i, 1H, CO–CH–CH2; g, 2H, –CH2), 1.16 (h, 3H, CO–NH–CH–CH3, CO–C–CH3) ppm (Fig. 2a).[NIPAM]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ETMA] [ETMA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BSPA] [BSPA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] [I] |
[PEGMA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [macro-CTA] [macro-CTA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] [I] |
Time (h) | GPCa | 1H NMR | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mn,GPCa (g mol−1) | PDI | Mn,NMRb (g mol−1) | NNIPAMc | NETMAc | NPEGMAc | ||||
| a Measured by GPC in DMF.b Obtained from 1H NMR.c Number of P(NIPAM-co-ETMA)-CTA and PPEGMA repeated units obtained from 1H NMR. | |||||||||
| A1-CTA | 660![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15 | 7900 | 1.17 | 7600 | 53 | 10 | ||
| A2-CTA | 630![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15 | 8300 | 1.13 | 9700 | 48 | 27 | ||
| A3-CTA | 560![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15 | 9000 | 1.21 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
42 | 35 | ||
| P1 | 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.45 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
111 | |||
| P2 | 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.24 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
113 | |||
| P3 | 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.31 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
114 | |||
Synthesis of the diblock copolymer P(NIPAM-co-ETMA)-b-P(PEGMA)
A typical synthetic procedure for P(NIPAM53-co-ETMA10)63-b-P(PEGMA)111 (P1) was as follows: A1-CTA (0.6 g, 0.08 mmol), AIBN (0.984 mg, 0.006 mmol), and PEGMA (1.6 g, 3.2 mmol) were dissolved in dry THF (15 mL). The following experimental procedure was similar to that for A1-CTA except that the reaction time was 20 h and the polymerization product was purified with ethyl ether (1.52 g, yield: 69%). Moreover, we have also synthesized P(NIPAM48-co-ETMA27)75-b-P(PEGMA)113 (P2) and P(NIPAM42-co-ETMA35)77-b-P(PEGMA)114 (P3) by a similar method, and the detailed process and characterization are shown in Table 1. 1H NMR (500 MHz, CDCl3, δ): 7.16–7.49 (f, 5H, Ph), 6.6 (a, 1H, CO–NH–CH(CH3)2), 4.1 (b, 1H, CO–N–CH(CH3)2), 3.9–4.2 (e, 2H, CH–CH2), 3.18–1.32 (d, 1H, SCH–; c, 2H, SCH–CH2; i, 1H, CO–CH–CH2; g, 2H, –CH2; m, 2H, –CH2), 1.17 (h, 3H, CO–NH–CH–CH3, CO–C–CH3), 3.67 (j, 2H, –CH2–OCH3), 3.4 (k, 3H, –CH2–OCH3), 0.88–1.02 (l, 3H, CO–C–CH3)53 ppm (Fig. 2b).Preparation of block copolymer micelles
In a typical experiment, block copolymer micelle solutions were prepared by dispersing 0.2 g of the polymers (P1, P2 and P3) in 40 mL of water. After vigorous stirring overnight at room temperature, the micelles were formed (denoted as M1, M2 and M3, respectively).Preparation of diblock copolymer-stabilized Au NPs (Au NPs@P)
Block copolymer-stabilized Au NPs with different molar feed ratios of [HAuCl4]/[P1] (MRs = 1/30, 1/10, 1/5, and 1/2, based on the ratios of Au atoms to sulfur atoms in the copolymers) were prepared by adding different amounts of the above block copolymer solution in the Au precursor (HAuCl4) solution under vigorous stirring. The concentration of Au in all the solutions is the same, at 121 μM. The samples from 1/30 to 1/2 represent the P1-capped Au NPs with the molar feed ratios (MRs). After 24 h under magnetic stirring, 100 μL NaBH4 (0.144 M) solution was quickly added, and the stirring was sustained for 24 h. The color of the solution changed from yellow to a wine color, showing the formation of Au NPs due to the reduction of gold ions by NaBH4 in the solution. The samples Au@P2 and Au@P3 with [HAuCl4]/[P1] = 1/5 were also obtained using the same experimental procedure. The solution was then dialyzed in double-distilled water for one week and the final solution was stored at 4 °C until use.Catalytic reaction of 4-NP using the block copolymer-stabilized Au NPs as catalysts
The catalytic activity measurements of the Au NPs@P systems are based on a catalytic reduction of 4-NP to 4-aminophenol (4-AP). In detail, different solutions of 4-NP (3 mL, 0.125 mM), NaBH4 (400 μL, 0.22 M) and Au NPs@P (20 μL, 121 μM) were added to a quartz cell as a substrate and incubated at a certain temperature in a water bath. In addition, the effects of different conditions, including MRs, content of PETMA segments (MR = 1/5) and the concentrations of reducing agents and catalysts on the catalytic activities of the systems were also studied using the same experimental procedure. Gold nanoparticles prepared by citric acid reduction were based on the literature54 and were used to perform the contrast experiment for the catalytic reduction of 4-NP. To study the effect of temperature on the catalytic activity, the temperature of the sample holder was controlled with an accuracy of 0.01 °C by an SYC-15G external circulating water bath. The catalytic activity of Au NPs@P1 at 20, 27, and 40 °C was measured.Measurements
Instrumental characterization
1H NMR spectra were obtained from an AVANCE Bruker spectrometer at basic frequencies of 500 MHz in CDCl3 and D2O solvents. Fourier transform infrared (FT-IR) spectra were recorded on a Magna 560 FT-IR spectrometer. UV-vis absorption spectra were recorded on a SHIMADZU UV-2550 UV-visible spectrophotometer in the range 200–800 nm. The molecular weights of the polymers were estimated by gel permeation chromatography (GPC) on a Waters instrument (Waters Corporation, USA) at a flow rate of 1.0 mL min−1 at 25 °C, using DMF as the eluent, and the molecular weights were determined vs. polystyrene standards. Transmission electron microscopy (TEM) was carried out on a JEM-2100F electron microscope. The particle size was calculated by measuring the diameters of samples from the corresponding TEM micrographs. The X-ray photoelectron spectroscopy (XPS) spectra were obtained from the surfaces with a diameter of 500 μm in area by means of a Quantum 2000 spectrometer using non-monochromatized Al Kα excitation radiation.Results and discussion
Synthesis and characterization of P(NIPAM-co-ETMA)-CTA and P(NIPAM-co-ETMA)-b-P(PEGMA)
The synthetic procedure for the preparation of P(NIPAM-co-ETMA)-CTA and P(NIPAM-co-ETMA)-b-P(PEGMA) via the RAFT polymerization method is shown in Scheme 1. The macromolecular chain transfer agent P(NIPAM-co-ETMA)-CTA was synthesized by copolymerization of NIPAM and ETMA using BSPA as the RAFT agent and AIBN as the initiator. Through changing the amount of the monomers, three P(NIPAM-co-ETMA)-b-P(PEGMA) block copolymers with different contents of PETMA segments were prepared. The following characterizations of FT-IR, 1H NMR and GPC can be used to demonstrate the successful synthesis of the block copolymers via RAFT polymerization.Fig. 1 shows the FT-IR spectra of A1-CTA, P1 and the Au precursor-containing P1 (Au3+@P1). A1-CTA displays a strong carbonyl absorption at 1653 cm−1 (amide I) and 1547 cm−1 (amide II) of the PNIPAM segments.55 The small peak at 1728 cm−1 is ascribed to the stretching vibration of carbonyl groups of PETMA segments, while the stretching vibration of the episulfide three-membered ring of the PETMA units can be observed at 615 cm−1.56 This observation indicates that A1-CTA has been successfully synthesized by RAFT copolymerization of NIPAM and ETMA. For P1 as shown in Fig. 1b, the characteristic peaks of carbonyl groups become stronger due to the stretching vibrations for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the ester groups of PPEGMA blocks. Clearly, the new peak that appeared at about 1112 cm−1 is assigned to the stretching vibrations for C–O–C in the ether groups of PPEGMA blocks, indicating the successful conjugation of PEGMA into P(NIPAM-co-ETMA)-CTA. In addition, the interaction between the episulfide groups in the PETMA segments and the Au precursors can be carefully confirmed by Fig. 1c. After the addition of the Au precursor, the peak at 615 cm−1 is considerably diminished due to the strong coordination interaction between the Au precursor and the episulfide group. This interaction can induce an episulfide opening reaction to form a sulfur–gold bond (S–Au3+) (Scheme S1, ESI†). Further evidence will be provided below.
O in the ester groups of PPEGMA blocks. Clearly, the new peak that appeared at about 1112 cm−1 is assigned to the stretching vibrations for C–O–C in the ether groups of PPEGMA blocks, indicating the successful conjugation of PEGMA into P(NIPAM-co-ETMA)-CTA. In addition, the interaction between the episulfide groups in the PETMA segments and the Au precursors can be carefully confirmed by Fig. 1c. After the addition of the Au precursor, the peak at 615 cm−1 is considerably diminished due to the strong coordination interaction between the Au precursor and the episulfide group. This interaction can induce an episulfide opening reaction to form a sulfur–gold bond (S–Au3+) (Scheme S1, ESI†). Further evidence will be provided below.
The chemical structure of P(NIPAM-co-ETMA)-CTA was ascertained by 1H NMR analysis as shown in Fig. 2a and the ESI (Fig. S1a and S2a†). The characteristic signals of the PNIPAM and PETMA segments are clearly observed at 6.4 (a), 4.0 (b) and 3.18 ppm (d). By comparing the integral area ratio of peaks d (the methine proton on the episulfide ring, –CH2CH(CH2)–S–) and e (the methine proton in the PNIPAM block, CO–N–CH(CH3)2), the repeating units of the PNIPAM block can be calculated to be 53. The repeating unit of PETMA is determined to be 10 for each initiate site. For the typical 1H NMR spectra (Fig. 2) of A1-CTA and P1, it can be seen that several new peaks appear in the spectrum of P1 (Fig. 2b) (for P2 and P3, see Fig. S1b and S2b, ESI†), except for the peaks ascribed to A1-CTA. The chemical shifts around 3.67 (j), 3.4 (k) and 0.78–0.93 ppm (l) are the characteristic signals of the PPEGMA blocks. Hence, the appearance of these new peaks demonstrates that the P(NIPAM-co-ETMA)-PPEGMA diblock copolymers have been successfully synthesized. To testify the occurrence of the episulfide opening reaction induced by gold ions in aqueous solution, the 1H NMR spectra of A1-CTA and Au3+@A1-CTA using D2O as a solvent are presented in the ESI (Fig. S3†). It can be clearly seen that there are characteristic signals of episulfide groups (d, 1H, SCH–; c, 2H, SCH–CH2) in Fig. S3a,† while the signals at these chemical shifts disappear and shift to other locations after the episulfide opening reaction induced by gold ions as shown in Fig. S3b,† which is direct evidence for the gold ion-induced episulfide opening reaction to form a sulfur–gold bond (S–Au3+).
The molecular weights of P1, P2 and P3 can be determined by GPC and 1H NMR, respectively. The molecular weight obtained from GPC testing is a relative value, which was calibrated with linear polystyrene standards. The molecular weight of the polymers and the lengths of the P(NIPAM-co-ETMA)-CTA and PPEGMA blocks were also determined on the basis of the 1H NMR integral area ratio of typical protons attributed to the P(NIPAM-co-ETMA)-CTA and P(PEGMA) blocks. The results of the molecular weights of the three block copolymers with different unit numbers of ETMA are summarized in Table 1. The measurements of molecular weights are in good agreement with the theoretical values predicted from the ratio of monomer-to-initiating sites, indicating the successful synthesis of P(NIPAM-co-ETMA)m-b-P(PEGMA)n (m ≈ 63, 75, 77, n ≈ 111, 113, 114). From the summary of Table 1, we can see that the composition of the P(NIPAM-co-ETMA) block in the diblock polymers with a similar length of PPEGMA blocks can be controlled by adjusting the molar ratio of monomers. Moreover, the polydispersity of the diblock polymers is relatively low. These results show that the RAFT polymerization is controlled well and the block copolymers are well defined.
The lower critical solution temperature (LCST) of P1-CTA is around 25 °C, determined from the temperature-dependent optical transmissions at 550 nm (Fig. 3), while the LCST of PNIPAM is around 33 °C.34 The LCST of P1-CTA is obviously lower than that of the pure PNIPAM, which can be explained by the hydrophobic interactions of the PNIPAM and PETMA segments. Moreover, the LCST of P1 is higher (about 30 °C) than that of P1-CTA, which should be attributed to the increased hydrophilicity of the polymer component with the incorporation of PEGMA blocks.35 As shown in Fig. S4 of the ESI,† the aqueous solution of P1 appears to be semi-transparent white after the phase transition with a higher temperature above 30 °C.
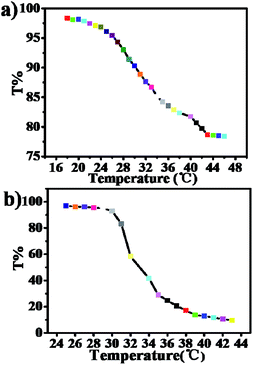 | ||
| Fig. 3 Temperature dependence of optical transmittance at 550 nm for A1-CTA (a) and P1 (b) aqueous solutions (sample concentration: 12 mg mL−1). | ||
TEM was used to investigate the morphology of the self-assembling aggregates when P1, P2 and P3 were dispersed in aqueous solution. It is clearly seen from Fig. 4a–c that polymer micelles (M1, M2 and M3) were formed by the direct self-assembly approach in aqueous solution because the block copolymers contain a certain amount of PETMA hydrophobic segments. For the P(NIPAM-co-ETMA)-b-P(PEGMA) diblock polymer, the PEGMA block can dissolve fully in water, while the PETMA segment has bad solubility. In this way, water becomes a solvent of selectivity to the diblock polymer. So the differences in the solubility of different segments become the main factor in judging whether there micelles are forming in the aqueous solution.57 In addition, these diblock copolymer self-assembled micelles are spherical in shape. Moreover, a relatively narrow size distribution was observed for M1, M2 and M3 with an average diameter of 20.8 ± 2.7, 25.6 ± 4.5 and 38.0 ± 8 nm, respectively (Fig. 4d–f). The micelles were found to have different sizes depending on the ratio of segments of PNIPAM and PETMA. When the ratio of segments of PNIPAM and PETMA is relatively small, the formed micelle has a large hydrophobic core. This can be explained by the fact that the P(NIPAM-co-ETMA) block with hydrophobic PETMA segments has bad solubility in water, which results in the P(NIPAM-co-ETMA) chains being entangled with each other to form large micelles,58 while there is a smaller effect of the external hydrophilic shell with a similar block length on the morphology of the micelles. So the micelle size is mainly related to the ratio of segments of PNIPAM and PETMA in the diblock copolymers.
Synthesis of block copolymer-stabilized Au NPs
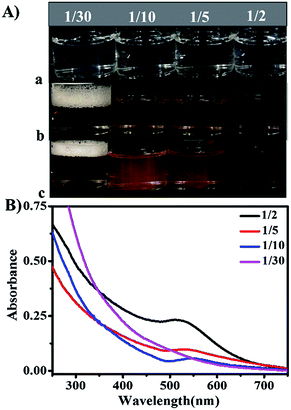
The block copolymer-stabilized Au NPs were obtained by in situ reduction of HAuCl4 using NaBH4 as the reduction agent in the presence of P(NIPAM-co-ETMA)-b-P(PEGMA) diblock copolymers. Here, the water-soluble PPEGMA block acts as the hydrophilic shell and the PNIPAM block with episulfide ligands and thermo-responsive properties is used as a stabilizer for the controlled synthesis of Au NPs. It has been reported by Egawa that the episulfide groups of PETMA segments would undergo a ring-opening reaction under acidic conditions and can effectively chelate heavy metal ions.43 Thus, as discussed above, the episulfide groups of ETMA units in the P(NIPAM-co-ETMA) block can provide a site to form the Au3+–S–ETMA complex in P(NIPAM-co-ETMA)-b-P(PEGMA) via an episulfide group ring-opening reaction because HAuCl4 is a strong acid.59 In this work, the block copolymer P1 was mainly selected to use as the stabilizer for the Au NPs in situ synthesis.The aqueous solution of the Au NPs@P1 systems containing HAuCl4 and P1 (MR = 1/2) was kept under magnetic stirring at room temperature to collect the changes in the UV-vis absorption spectra of different time periods (Fig. 5). NaBH4 is a strong reducing agent that leads to a fast nucleation rate and smaller gold nanoparticles. Fig. 5 shows the typical surface plasmon resonance at about 518 nm, indicating the formation of Au NPs.15 Moreover, the UV-vis absorption spectra (Fig. 5) collected from the solution have no obvious change in a typical position of the SPR maximum by increasing the reaction time. This result indicates that 1 h duration is enough for the reduction of NaBH4 in the mixture of HAuCl4 and block copolymer P1. Fig. 6A-a shows the photographs of the mixed aqueous solutions of HAuCl4 and the block copolymer P1 with different MRs left at room temperature for 0 h. When NaBH4 is added to Au@P1 systems, the solution immediately turns to a red wine color (Fig. 6A-b) and after magnetic stirring for 24 h at room temperature, further chemical reduction has occurred (Fig. 6A-c). Fig. 6B shows that different MRs of the Au@P1 systems correspond to different absorption wavelengths, suggesting that the gold nanoparticles have different sizes.11,60 In addition, we found that there is no obvious absorption at 520 nm for Au@P1 with MR = 1/30, which may be attributed to the formation of smaller gold nanoclusters.61
 | ||
| Fig. 5 UV-vis absorption spectra of Au@P1 (MR = 1/2) aqueous solution prepared at different time periods with added NaBH4 to the Au@P1 system. | ||
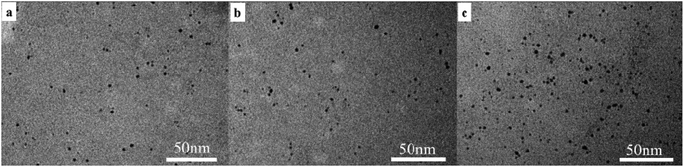
The specific size and morphology of Au NPs@P1 with different MRs can be obtained from the TEM images in Fig. 7. The clear spherical micelle morphology is observed for the sample with MR = 1/30, and the micelle size is about 42 ± 21 nm (Fig. S5a, ESI†). It can be seen that the size of the micelle is obviously bigger than that of the pure M1 micelle because HAuCl4 is absorbed on the core of the P(NIPAM-co-ETMA) blocks and made the structure of the micelles become dense. The P(NIPAM-co-ETMA) block with episulfide ligands gradually forms the core due to the induction of HAuCl4, while the PPEGMA block remains hydrophilic and forms the hydrophilic shell. This indicates that the gold nanoparticles are well dispersed in the micelles and the size is very small. In all cases, the gold nanoparticles formed by NaBH4 reduction are uniformly distributed in the core of the micelles. Although single Au NPs cannot be seen, we can observe the crystalline lattice fringes of gold nanoparticles from the high resolution TEM image (yellow mark in the inset of Fig. 7a). The TEM images show that Au NPs@P1 forms different morphologies on further increasing the MR (Fig. 7b–d). With the MR increasing from 1/10 and 1/5 to 1/2, the content of HAuCl4 in all the solutions is the same but the block copolymers coated with Au NPs are gradually reduced. The micelle morphology of the Au NPs encapsulated by polymeric micelles becomes less obvious. This result indicates that the micelles of the polymers were disassembled when the concentration of the Au precursors increases (or MRs increase) in the micelle solution. So the morphology of the thin polymer layer-coated Au NPs becomes apparent. One can see from Fig. S5b–d in the ESI† that the Au NPs have an average particle diameter of 1.3 ± 0.4 nm, 1.9 ± 0.5 nm and 6.5 ± 2 nm, respectively. The Au NPs show an increased size from 1.3 to 6.5 nm with the increasing MRs. The high resolution TEM images in the inset of Fig. 7a and d further reveal that the Au NPs have a crystalline lattice fringe of 0.206 nm (yellow mark in Fig. 7a) and 0.238 nm (yellow mark in Fig. 7d), corresponding to the primary reflection of the (200) and (111) lattice of Au, respectively. Therefore, it is easy to infer that the higher the P1 concentration is, the more effective the capping function of P1 is, and the more nucleation sites P1 provides to result in smaller Au NPs finally. Similar results have also been reported in previous work.62 These results, on the other hand, further reveal the important role of the diblock copolymers in the in situ generation of gold nanoparticles.
 | ||
| Fig. 7 Representative TEM images of Au NPs@P1 prepared at the different MRs of 1/30 (a), 1/10 (b), 1/5 (c) and 1/2 (d). | ||
Fig. 8 shows the TEM images of Au NPs@P1, Au NPs@P2 and Au NPs@P3. We can see that there is no large change in the size and the size distribution (Fig. S6, ESI†) of the gold nanoparticles. The TEM image and diameter distribution histogram of the control sample of Au NPs@citrate are also shown in Fig. S7b of the ESI.† The average diameter of the Au NPs is 10.4 ± 1.1 nm. Au NPs@citrate exhibits signs of aggregation or clumping in Fig. S7a,† whereas Au NPs@P1, Au NPs@P2 and Au NPs@P3 present a well-dispersed fashion regardless of the content of PETMA segments as shown in Fig. 8. Furthermore, we attempted to prepare the Au NPs without addition of P1. It was found that a lot of precipitation soon appeared in the aqueous solution of gold ions after the fresh aqueous solution of NaBH4 was added. Therefore, we believe that gold nanoparticles should be present within the polymers for the block copolymer-stabilized Au NPs. No precipitate and obvious color change is observed for a long period of three months, indicating the good stability of the block copolymer-stabilized Au NPs, and the hydrophobic PETMA domains still endowed the nanoparticles with a high colloidal stability.
The composition of the formed Au NPs@P1 hybrid was checked further using XPS. Fig. 9 represents the XPS spectrum of the P1-stabilized Au NPs (MR = 1/2), and only Au(0) peaks are observed at binding energies of 87.3 eV (Au-4f5/2) and 83.7 eV (Au-4f7/2).63 Therefore, Au(III) can be reduced to Au(0) when the strong reducing agent NaBH4 is added to the mixture solution of HAuCl4 and the block copolymer P1. The XPS spectra of S 2p of P1 and Au NPs@P1 are shown in Fig. S8 of the ESI.† According to the literature report, the peak at 164 eV corresponds to non-oxidized sulfur (episulfide) and the 2p profiles can be fitted using a splitting value of 1.2 eV.64,65 The peaks of S 2p3/2 and S 2p1/2 for the episulfide groups of the PETMA segments are observed at binding energies of 163.1 and 164.3 eV, respectively (Fig. S8a, ESI†). For Au NPs@P1 (MR = 1/2), two sets of peaks corresponding to S 2p3/2 and S 2p1/2 are observed at the binding energies of 162.9 and 164.1 eV, respectively (Fig. S8b, ESI†).65,66 It seems that there is no obvious change in the XPS spectrum, but the peaks of S 2p slightly shift to a low binding energy on the whole. The above discussions about the FT-IR and 1H NMR spectra have demonstrated the formation of a sulfur–gold bond (S–Au3+) by the gold ion-induced episulfide opening reaction. So, this observation should be attributed to the fact that the sulfur–gold bonds formed by the episulfide opening reaction also have XPS peaks which are very similar to that of S 2p of the episulfide groups at binding energies between 162 and 164 eV.64–66
Catalytic ability of the block copolymer-stabilized Au NPs for 4-NP reduction
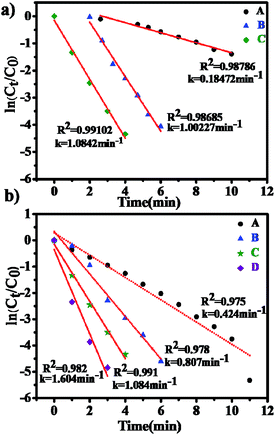
The catalytic activity of Au NPs@P1 (MR = 1/2) was studied through a well-informed model reaction, which is the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) in the presence of NaBH4. Initially, the 4-NP solution has a bright yellow color, which turns yellow-green upon the addition of NaBH4. In addition, the absorption peak of 4-NP immediately undergoes a red shift from 317 to 400 nm due to the formation of 4-nitrophenolate ions in alkaline conditions by the action of NaBH4 (Fig. S9, ESI†).67 As shown in Fig. S10 of the ESI,† without addition of the catalyst Au NPs@P1 (MR = 1/2), the absorption peak at 400 nm remains undiminished for a long time, demonstrating that the reducing agent NaBH4 itself is unable to reduce the 4-nitrophenolate ion. However, with the use of Au NPs@P1, the dark yellow color gradually became colorless with the progression of the reduction to 4-AP (Fig. S11, ESI†). The kinetics of the reaction can be intermittently monitored using UV-vis spectroscopy. Specifically, as seen in Fig. 10, the decrease in intensity of the UV absorption at 400 nm in a very short timescale is an indication of the good catalytic activity of Au NPs@P in the reduction of 4-NP. In addition, the peak at 317 nm can be attributed to the production of 4-AP. The rate constant (k) of the reaction can be determined from the plot of ln(Ct/C0) vs. time.26,68 There exists a clear isosbestic point between the two absorption bands in the UV spectra, indicating that the two primary species are responsible for the conversion reaction. On the basis of the UV-vis spectra, therefore, pseudo-first-order reaction kinetics is applied to determine the rate constant k for the reaction.18 In order to investigate the influence of the different P(NIPAM-co-ETMA)-b-P(PEGMA) block copolymers (P1, P2, P3) on the catalytic activity of Au NPs, the reaction process was tracked by UV-vis measurement as shown in Fig. 10. The reaction was analyzed by a first-order rate law due to the fact that an excess of NaBH4 (400 μL) was used and an excess of catalyst Au NPs@P (MR = 1/5, 300 μL) was assumed to be present. Additionally, we found that the catalytic activity of Au NPs@P decreased with the increasing ratio of PETMA and PNIPAM segments. For Au NPs@P-catalyzed reaction systems, the reaction finished in 6, 12, and 16 min with k = 0.312, 0.407, and 0.907 min−1 for Au NPs@P1, Au NPs@P2, and Au NPs@P3, respectively. It was thought that the reduction reaction might be determined by the diffusion of the polar reactants of 4-NP and BH4− into the surface of Au NPs and the product of 4-AP from the surface.62 For Au NPs@P catalytic systems, the presence of the hydrophobic PETMA segments with a high polarity effect should be an important factor that may impede the diffusion of reactants and products on the surface of the Au NPs.16 Because the thickness of the PETMA segments attached to the surface of the Au NPs increases from P1 and P2 to P3 with the increasing ratio of PETMA and PNIPAM segments, this observation further showed that the catalytic activity of the Au NPs should be dominated by diffusion.We choose Au NPs@P1 (MR = 1/2) as the catalyst to conduct several control experiments to find out the optimum catalytic reduction reaction conditions of 4-NP. Furthermore, the effect of the concentration of catalyst and reductant on the reduction rate was studied when keeping the reaction temperature constant (Fig. 11). From the linear relations of ln(Ct/C0) with time, we can determine the rate constant (k) at 27 °C to be 0.185, 1.002, 1.084 min−1 with different concentrations of reductant (Fig. 11a) and k is 0.424, 0.807, 1.084 and 1.604 min−1 with different concentrations of catalysts (Fig. 11b). It can be found that the value of k shows a linear increase with the increasing concentration of catalyst and reductant. Similar results have also been found in other research systems.18,26,69,70 These results are comparable or superior to those reported previously. For example, Au NPs on the surface of poly(2-(dimethylamino)ethyl methacrylate) grafted onto a solid polystyrene core showed a k value of 0.192 min−1 at 436 μM of Au NPs.71 β-D-Glucose-stabilized Au NPs presented a k value of 0.392 min−1 using 200 μM of Au NPs.72 Poly(amidoamine)-based hollow capsule-stabilized Au NPs presented a rate constant of 0.12 min−1.73 Although k is lower in all of our samples for the reduction system containing 20 μL of Au NPs@P1 (MR = 1/2) with 400 μL of NaBH4, this Au NPs@P1 system still shows a high catalytic activity for the reduction of 4-NP as compared to the above reports. In addition, it is noted that Au NPs@P1 as a catalyst displays a superior catalytic activity (k = 0.424 min−1) in the reduction of 4-NP as compared to that of the Au NPs prepared by citrate reduction (k = 0.134 min−1) with an average diameter of 10.4 ± 1.1 nm (Fig. S7c, ESI†). This observation shows that not only the Au NPs but also the surrounding P1 play a critical role in the enhancement of the reduction efficiency.
Here, the catalytic efficiency of Au NPs@P1 was also tested using four samples with different MRs of 1/30, 1/10, 1/5 and 1/2 (Fig. 12). The different catalytic times are obtained when their gold content is the same, indicating that the catalytic efficiency is different with the different MRs of Au NPs@P1. It is found that the catalytic activity of Au NPs@P1 increases with the increase of the MRs. In addition, the reduction time of the catalyst is shorter with the increasing size of the Au NPs (Fig. 7), and the Au NPs with a smaller size have a slower reduction rate. Similar results have also been found in other research systems.41,62,74 As is known, the metal NPs catalyze this reaction by facilitating electron relay from the donor BH4− to acceptor 4-NP to overcome the kinetic barrier.62 The rate of electron transfer at the metal surface can be influenced by two aspects: (1) diffusion of 4-NP to the metal surfaces, and (2) interfacial electron transfer and diffusion of 4-AP away from the surface.62 Thus, the diffusion of 4-NP and the particle size should mainly determine the rate of the reduction. The adsorbing polymer chains would affect the diffusion of 4-NP to the surface of the metal nanoparticles. Thus, from this point, the high catalytic efficiency of Au NPs@P1 (MR = 1/2) should be attributed to the least coating amount of P1 on the surface of Au NPs as compared with the samples with the low MRs of 1/30, 1/10 and 1/5. In addition, the Au NPs for Au NPs@P1 (MR = 1/2) have an average particle diameter of 6.5 ± 1 nm. Previous studies have shown that the gold nanoparticles of about 8 nm in diameter exhibited a relatively high catalytic efficiency.41,62,74,75 Therefore, the block copolymer P1 can be used as a stabilizer in the preparation of Au NPs@P1 (MR = 1/2) with a high catalytic activity for the reduction of 4-NP by NaBH4.
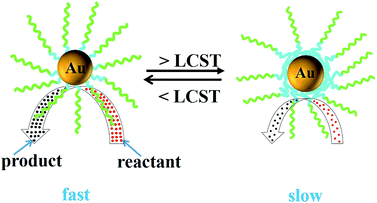
The PNIPAM chains in the block copolymers can change their position with the shrinking and extension of the PNIPAM chains under thermal stimuli.40 So the thermo-responsive catalytic behavior of Au NPs@P1 as a catalyst was also investigated by UV-vis absorption spectroscopy. The catalytic systems containing Au NPs@P1 (MR = 1/2) were subjected to an increasing temperature from 20 to 40 °C. Fig. 13 shows the values of k at different temperatures. Clearly, the value of k increases with the increasing temperature in the range from 20 to 27 °C, indicating that the rate of the reduction reaction can be enhanced by increasing the temperature. This tendency follows the typical dependence of the rate constant on temperature described by the Arrhenius equation and is similar to a general catalyst. It has been reported that the reduction rate and therefore the value of k increases with the increase in temperature76–78 when the reduction is performed at a temperature below the LCST of PNIPAM. As shown in Scheme 3, the possible reason is that the corona-forming PNIPAM chains are hydrophilic and therefore the reactants of 4-NP and NaBH4 can easily diffuse through the corona layer of PNIPAM to reach the surface of the gold nanoparticles to arouse the reduction when the reduction is performed at a temperature below the LCST of the thermo-responsive polymers.22 Whereas, when temperature is further increased to above the LCST, the value of k decreased with the increase in temperature until reaching a constant at about 40 °C. The abnormal decrease in the value of k is possibly due to the fact that the enhancement of temperature would lead to the hydrophobicity of PNIPAM and the PNIPAM chains collapsing to form a hydrophobic barrier on the gold nanoparticles at this temperature, which decelerates the access of the reactants to the gold nanoparticles and therefore decreases the reaction rate (Scheme 3). Thus, the thermo-responsive PNIPAM chains are just like a switch which controls the reaction by adjusting the temperature.
Conclusions
Well-defined novel thermo-responsive diblock copolymers P(NIPAM-co-ETMA)-b-P(PEGMA) were synthesized by RAFT polymerization. This type of block copolymer in aqueous solution can directly self-assemble into micelles with hydrophobic P(NIPAM-co-ETMA) blocks as the core. The block copolymers containing episulfide groups were used as stabilizers for the in situ preparation of Au NPs for thermo-responsive catalytic reduction of 4-NP. Experiments showed that the block copolymer-stabilized Au NPs exhibited excellent colloidal stability, and the size of the in situ formed Au NPs could be controlled by the different MRs. In addition, we used the synthesized diblock copolymer-stabilized Au NPs as a model to investigate the influence of the content of PETMA segments and different MRs on the catalytic activity of Au NPs. The obtained kinetic data showed that the catalytic activity was closely related to the limitation of the reactant diffusion. Thus, the catalytic activity of Au NPs@P increased with the decreasing content of PETMA segments in the block copolymers. For different MRs, the catalytic activity of Au NPs@P1 was different, and Au NPs@P1 (MR = 1/2) exhibited a higher catalytic activity. The constructed Au NPs@P could act as a thermo-responsive catalyst and the catalytic activity could be adjusted by the thermo-responsive phase transition of the PNIPAM blocks. Thus, the thermo-responsive PNIPAM chains played a role in the control of the catalytic rate. Our study shows that the diblock copolymer plays a vital role in the process of preparing gold nanoparticles with high catalytic activity and good stability. Therefore, the novel block copolymers containing episulfide ligands may find many potential applications for stabilization of metal nanoparticles for catalytic and other applications.Acknowledgements
We would like to appreciate the financial support of the National Natural Science Foundation of China (21574017) and Jilin Provincial Department of Education.Notes and references
- Y. Zhao, Y. Zheng, C. Zhao, J. You and F. Qu, Biosens. Bioelectron., 2015, 71, 200–206 CrossRef CAS PubMed.
- A. Corma and H. Garcia, Chem. Soc. Rev., 2008, 37, 2096–2126 RSC.
- M.-C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS PubMed.
- B. Le Droumaguet, R. Poupart and D. Grande, Polym. Chem., 2015, 6, 8105–8111 RSC.
- F. Vatansever, W. C. M. A. de Melo, P. Avci, D. Vecchio, M. Sadasivam, A. Gupta, R. Chandran, M. Karimi, N. A. Parizotto and R. Yin, FEMS Microbiol. Rev., 2013, 37, 955–989 CrossRef CAS PubMed.
- S. Eustis and M. A. El-Sayed, Chem. Soc. Rev., 2006, 35, 209–217 RSC.
- O. V. Salata, J. Nanobiotechnol., 2004, 2, 3 CrossRef PubMed.
- H. Otsuka, Y. Nagasaki and K. Kataoka, Adv. Drug Delivery Rev., 2012, 64, 246–255 CrossRef.
- F. Kretschmer, S. Mühlig, S. Hoeppener, A. Winter, M. D. Hager, C. Rockstuhl, T. Pertsch and U. S. Schubert, Part. Part. Syst. Charact., 2014, 31, 721–744 CrossRef.
- Y. Mei, Y. Lu, F. Polzer, M. Ballauff and M. Drechsler, Chem. Mater., 2007, 19, 1062–1069 CrossRef CAS.
- A. V. Gaikwad, P. Verschuren, E. Eiser and G. Rothenberg, J. Phys. Chem. B, 2006, 110, 17437–17443 CrossRef CAS PubMed.
- C. Burda, X. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025–1102 CrossRef CAS PubMed.
- J. Tian, G. Liu, C. Guan and H. Zhao, Polym. Chem., 2013, 4, 1913–1920 RSC.
- J. Shan and H. Tenhu, Chem. Commun., 2007, 4580–4598 RSC.
- F. Gröhn, B. J. Bauer, Y. A. Akpalu, C. L. Jackson and E. J. Amis, Macromolecules, 2000, 33, 6042–6050 CrossRef.
- Y. Que, C. Feng, S. Zhang and X. Huang, J. Phys. Chem. C, 2015, 119, 1960–1970 CAS.
- F. Goto, K. Ishihara, Y. Iwasaki, K. Katayama, R. Enomoto and S.-i. Yusa, Polymer, 2011, 52, 2810–2818 CrossRef CAS.
- E. Seo, J. Kim, Y. Hong, Y. S. Kim, D. Lee and B.-S. Kim, J. Phys. Chem. C, 2013, 117, 11686–11693 CAS.
- S. Förster and M. Antonietti, Adv. Mater., 1998, 10, 195–217 CrossRef.
- Y. Kotsuchibashi, M. Ebara, T. Aoyagi and R. Narain, Polym. Chem., 2012, 3, 2545–2550 RSC.
- H. H. Nguyen, A. Brulet, D. Goudouneche, P. Saint-Aguet, N. Lauth-de Viguerie and J. D. Marty, Polym. Chem., 2015, 6, 5838–5850 RSC.
- Y. Wang, G. Wei, W. Zhang, X. Jiang, P. Zheng, L. Shi and A. Dong, J. Mol. Catal. A: Chem., 2007, 266, 233–238 CrossRef CAS.
- L. M. Bronstein, S. N. Sidorov, P. M. Valetsky, J. Hartmann, H. Cölfen and M. Antonietti, Langmuir, 1999, 15, 6256–6262 CrossRef CAS.
- H. Yabu, T. Jinno, K. Koike, T. Higuchi and M. Shimomura, Macromolecules, 2011, 44, 5868–5873 CrossRef CAS.
- J. Li, J. Liang, W. Wu, S. Zhang, K. Zhang and H. Zhou, New J. Chem., 2014, 38, 2508–2513 RSC.
- X. Chen, D. Zhao, Y. An, L. Shi, W. Hou and L. Chen, J. Nanopart. Res., 2010, 12, 1877–1887 CrossRef CAS.
- C. Maiti, R. Banerjee, S. Maiti and D. Dhara, Langmuir, 2014, 31, 32–41 CrossRef PubMed.
- Y. Bao, G. Shen, H. Liu and Y. Li, Polymer, 2013, 54, 652–660 CrossRef CAS.
- A. E. Di Mauro, M. Striccoli, N. Depalo, E. Fanizza, L. Cano, C. Ingrosso, A. Agostiano, M. L. Curri and A. Tercjak, Soft Matter, 2014, 10, 1676–1684 RSC.
- P.-L. Kuo, C.-C. Chen and M.-W. Jao, J. Phys. Chem. B, 2005, 109, 9445–9450 CrossRef CAS PubMed.
- S. N. Sidorov, L. M. Bronstein, P. M. Valetsky, J. Hartmann, H. Cölfen, H. Schnablegger and M. Antonietti, J. Colloid Interface Sci., 1999, 212, 197–211 CrossRef CAS PubMed.
- M. I. Gibson and R. K. O’Reilly, Chem. Soc. Rev., 2013, 42, 7204–7213 RSC.
- N. Yan, J. Zhang, Y. Yuan, G. T. Chen, P. J. Dyson, Z. C. Li and Y. Kou, Chem. Commun., 2010, 46, 1631–1633 RSC.
- H. Wei, S.-X. Cheng, X.-Z. Zhang and R.-X. Zhuo, Prog. Polym. Sci., 2009, 34, 893–910 CrossRef CAS.
- L. Hou and P. Wu, Soft Matter, 2015, 11, 2771–2781 RSC.
- M. Beija, J.-D. Marty and M. Destarac, Chem. Commun., 2011, 47, 2826–2828 RSC.
- D. Li, Q. He and J. Li, Adv. Colloid Interface Sci., 2009, 149, 28–38 CrossRef CAS PubMed.
- J. Zhang, M. Zhang, K. Tang, F. Verpoort and T. Sun, Small, 2014, 10, 32–46 CrossRef CAS PubMed.
- M. Beija, E. Palleau, S. Sistach, X. Zhao, L. Ressier, C. Mingotaud, M. Destarac and J. D. Marty, J. Mater. Chem., 2010, 20, 9433–9442 RSC.
- T. Yin, X. Liu, J. Wang, Y. An, Z. Zhang and L. Shi, RSC Adv., 2015, 5, 47458–47465 RSC.
- Y. Dai, Y. Li and S. Wang, J. Catal., 2015, 329, 425–430 CrossRef CAS.
- A. Q. Zhang, L. J. Cai, L. Sui, D. J. Qian and M. Chen, Polym. Rev., 2013, 53, 240–276 CrossRef CAS.
- H. Egawa, Anal. Chim. Acta, 1984, 162, 339–346 CrossRef.
- F. E. Rogers, J. Polym. Sci., Part A: Polym. Chem., 1965, 3, 2701–2703 CAS.
- T. Nonaka, E. Noda and S. Kurihara, J. Appl. Polym. Sci., 2000, 77, 1077–1086 CrossRef CAS.
- T. Nonaka, S. Matsumura, T. Ogata and S. Kurihara, J. Membr. Sci., 2003, 212, 39–53 CrossRef CAS.
- L. Wu, U. Glebe and A. Boker, Polym. Chem., 2015, 6, 5143–5184 RSC.
- R. K. áO’Reilly, Chem. Commun., 2008, 4183–4185 Search PubMed.
- T. Nonaka and K. Fujita, J. Membr. Sci., 1998, 144, 187–195 CrossRef CAS.
- L. Y. Li, W. D. He, W. T. Li, K. R. Zhang, T. T. Pan, Z. L. Ding and B.-Y. Zhang, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 5018–5029 CrossRef CAS.
- Y. Zhao, X. Shi, H. Gao, L. Zhang, F. Zhu and Q. Wu, J. Mater. Chem., 2012, 22, 5737–5745 RSC.
- M. L. Tebaldi de Sordi, M. A. Ceschi, C. L. Petzhold and A. H. E. Müller, Macromol. Rapid Commun., 2007, 28, 63–71 CrossRef CAS.
- B. P. Koiry, A. Chakrabarty and N. K. Singha, RSC Adv., 2015, 5, 15461–15468 RSC.
- B. V. Enustun and J. Turkevich, J. Am. Chem. Soc., 1963, 85, 3317–3328 CrossRef CAS.
- Y. Tang, L. Liu, J. Wu and J. Duan, J. Colloid Interface Sci., 2013, 397, 24–31 CrossRef CAS PubMed.
- C. Lü, Z. Cui, Y. Wang, B. Yang and J. Shen, J. Appl. Polym. Sci., 2003, 89, 2426–2430 CrossRef.
- P. Khullar, A. Mahal, V. Singh, T. S. Banipal, G. Kaur and M. S. Bakshi, Langmuir, 2010, 26, 11363–11371 CrossRef CAS PubMed.
- A. Mei, X. Guo, Y. Ding, X. Zhang, J. Xu, Z. Fan and B. Du, Macromolecules, 2010, 43, 7312–7320 CrossRef CAS.
- Z. Luo, V. Nachammai, B. Zhang, N. Yan, D. T. Leong, D. E. Jiang and J. Xie, J. Am. Chem. Soc., 2014, 136, 10577–10580 CrossRef CAS PubMed.
- V. Amendola and M. Meneghetti, J. Phys. Chem. C, 2009, 113, 4277–4285 CAS.
- M. Nawaz, I. Ud-Din, G. Price and M. K. Baloch, J. Polym. Res., 2014, 21, 1–7 CAS.
- Y. Liu, L. Liu, M. Yuan and R. Guo, Colloids Surf., A, 2013, 417, 18–25 CrossRef CAS.
- T. F. Jaramillo, S.-H. Baeck, B. R. Cuenya and E. W. McFarland, J. Am. Chem. Soc., 2003, 125, 7148–7149 CrossRef CAS PubMed.
- E. Humeres, K. M. de Castro, R. d. F. P. M. Moreira, W. H. Schreiner, A. E. Aliev, M. Canle, J. A. Santaballa and I. Fernández, J. Phys. Org. Chem., 2008, 21, 1035–1042 CrossRef CAS.
- H. A. Almukhlifi and R. C. Burns, Appl. Catal., A, 2015, 502, 174–187 CrossRef CAS.
- A. Boccia, R. Zanoni, A. Arduini, L. Pescatori and A. Secchi, Surf. Interface Anal., 2012, 44, 1086–1090 CrossRef CAS.
- Y.-C. Chang and D. H. Chen, J. Hazard. Mater., 2009, 165, 664–669 CrossRef CAS PubMed.
- A. Murugadoss and A. Chattopadhyay, Nanotechnology, 2008, 19, 015603 CrossRef CAS PubMed.
- Y. Lu, Y. Mei, M. Ballauff and M. Drechsler, J. Phys. Chem. B, 2006, 110, 3930–3937 CrossRef CAS PubMed.
- X. Chen, D. Zhao, Y. An, Y. Zhang, J. Cheng, B. Wang and L. Shi, J. Colloid Interface Sci., 2008, 322, 414–420 CrossRef CAS PubMed.
- M. Zhang, L. Liu, C. Wu, G. Fu, H. Zhao and B. He, Polymer, 2007, 48, 1989–1997 CrossRef CAS.
- J. Liu, G. Qin, P. Raveendran and Y. Ikushima, Chem.–Eur. J., 2006, 12, 2131–2138 CrossRef CAS PubMed.
- H. Wu, Z. Liu, X. Wang, B. Zhao, J. Zhang and C. Li, J. Colloid Interface Sci., 2006, 302, 142–148 CrossRef CAS PubMed.
- R. Fenger, E. Fertitta, H. Kirmse, A. F. Thunemann and K. Rademann, Phys. Chem. Chem. Phys., 2012, 14, 9343–9349 RSC.
- S. Panigrahi, S. Basu, S. Praharaj, S. Pande, S. Jana, A. Pal, S. K. Ghosh and T. Pal, J. Phys. Chem. C, 2007, 111, 4596–4605 CAS.
- Y. Wang, G. Wei, F. Wen, X. Zhang, W. Zhang and L. Shi, J. Mol. Catal. A: Chem., 2008, 280, 1–6 CrossRef CAS.
- C. L. Forryan and R. G. Compton, Phys. Chem. Chem. Phys., 2003, 5, 4226–4230 RSC.
- S. P. Bawane and S. B. Sawant, Appl. Catal., A, 2005, 293, 162–170 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra02885c |
| This journal is © The Royal Society of Chemistry 2016 |