The effect of glassing solvent deuteration and Gd3+ doping on 13C DNP at 5 T
Andhika Kiswandhi†
a,
Bimala Lama†b,
Peter Niedbalskia,
Mudrekh Goderyaa,
Joanna Longb and
Lloyd Lumata*a
aDepartment of Physics, University of Texas at Dallas, Richardson, Texas 75080, USA. E-mail: lloyd.lumata@utdallas.edu
bDepartment of Biochemistry and Molecular Biology, University of Florida, Gainesville, FL 32610, USA
First published on 14th April 2016
Abstract
We report the influence of glassing solvent deuteration and Gd3+ doping on 13C dynamic nuclear polarization (DNP) nuclear magnetic resonance (NMR) performed on [1-13C] sodium acetate at B0 = 5 T and 1.2 K. Our data reveal that at 5 T, glassing solvent deuteration still results in a 40% improvement of the 13C DNP signal when a large electron spin resonance (ESR) linewidth 4-oxo-TEMPO free radical is used, but results in a 60% decrease of the DNP signal in the case of a sample doped with small ESR linewidth trityl OX063. An addition of a trace amount of the Gd3+ complex Gd–HP–DO3A led to a negligible slight decrease on the 13C polarization TEMPO-doped sample, but is still relatively beneficial for the trityl-doped sample with 30% improvement of the DNP-enhanced 13C polarization. These findings indicate that while these DNP optimization steps are still valid at 5 T, the effects are not as pronounced as observed in 13C DNP at B0 = 3.35 T. These DNP results at 5 T are discussed thermodynamically within the framework of the thermal mixing model of DNP.
1. Introduction
Conventional nuclear magnetic resonance (NMR) spectroscopy, while very useful and widely used, lacks sensitivity due to the small difference in the spin populations between nuclear Zeeman spin states at ambient conditions, resulting in low polarization of the nuclei. In other words, this low polarization level stems from the energy difference between the two nuclear-spin states being low compared to the thermal energy.1 In contrast, electron spins can be polarized rather easily, owing to their higher gyromagnetic ratio γ and thus a larger energy gap between the two spin states. In general, the γ of electrons is about three orders of magnitude higher than that of typically observed nuclei.Dynamic nuclear polarization (DNP) is a method of amplifying nuclear polarization by transferring the high polarization of electrons to the nuclei via microwave radiation at low temperature and high magnetic field.2 The resulting large enhancement in the nuclear polarization or NMR sensitivity leads to time savings in NMR measurements, especially on nuclei having low gyromagnetic ratios such 13C, 15N, 89Y, or 107,109Ag.2–8 Furthermore, DNP-NMR has become a rapidly emerging technique in the biomedical research community, where it is used in probing metabolic anomalies in various diseases such as cancer, with the combined superb sensitivity and high specificity provided by hyperpolarized 13C NMR spectroscopy or imaging (MRI).9–14
In a typical dissolution DNP setup,2 a sample containing the target nuclei is doped with a free radical at an optimum concentration and embedded uniformly in a glassing matrix. The whole solution is then cooled down to a cryogenic temperature (<4 K) in a high magnetic field (>1 T). The purpose of this step is to polarize the electrons provided by the free radicals by Boltzmann thermal polarization. Hyperpolarization is achieved by irradiating the frozen sample with a microwave frequency close to the electron spin resonance (ESR) frequency of the free radical. This process results in a transfer of the high spin alignment from the electrons to the target nuclei.1,15,16 Taking advantage of the relatively long spin-lattice relaxation time T1 of target nuclei, dissolution DNP is able to harness the amplified NMR signals in the liquid-state at physiologically tolerable temperatures.2
Based on the procedure above, for any nuclei, two factors contributing to the sensitivity enhancement can be identified: (i) instrumental setup (B0/T ratio) and (ii) sample preparation. Factor (i) determines the polarization of the electrons in the free radical; B0 also determines the energy difference between the nuclear Zeeman energy levels or the Larmor frequency of the nuclei (ωI). B0 and T also determine the relaxation constants of the electron and nuclear spins. Factor (ii), in particular the type of free radical used, determines whether the width (D) of the electron spin resonance (ESR) spectrum of the free radical is larger or smaller than ωI. Together, these factors determine the way by which the predominant DNP mechanism proceeds: (a) via the solid effect when D < ωI or (b) via the thermal mixing effect which occurs when D > ωI.1,15,16 A study by Lumata et al. has shown the effects of deuteration of the glassing matrix in 13C DNP at 3.35 T, which improves the nuclear polarization when galvinoxyl, DDPH, or 4-oxo-TEMPO is used.17 However, the glassing solvent deuteration decreases the nuclear polarization when BDPA or trityl OX063 was used as the free radical dopant.17 Similar studies on the effects of addition of trace amounts of lanthanides such as Gd3+ on DNP samples reported significant improvements in the DNP-enhanced polarization levels.18–21 Many DNP optimization studies reported previously were performed at magnetic fields of 3.35 T, mainly due to the availability of commercial hyperpolarizer operating at 3.35 T (Hypersense, Oxford Instruments, UK) and W-band microwave source. Recent work has shown greater DNP-enhanced polarization levels can be gained at fields of 5–7 T22–25 and a recent DNP hyperpolarizer for clinical purposes (SPINlab™, GE Healthcare, WI) has an operating field of 5 T.26 Therefore, greater exploration of suitable sample conditions for optimal polarization properties at these fields are needed, especially as the electronic properties of free radicals change with field.27 In this article, we have investigated the influence of glassing solvent deuteration and the inclusion of Gd3+ on 13C DNP at a field of 5 T. Free radicals 4-oxo-TEMPO and trityl OX063 (Fig. 1) are used to represent free radicals with wide and narrow ESR linewidths, respectively.
 | ||
| Fig. 1 Structures of ProHance Gd3+ contrast agent and free radical polarizing agents studied in this work. | ||
2. Experimental
2.1 Materials
The free radicals were obtained from commercial suppliers: (a) tris[8-carboxyl-2,2,6,6-benzo(1,2-d:5d)-bis(1,3)dithiole-4-yl]methyl sodium salt (trityl OX063) [Oxford Instruments Molecular Biotools], (b) 4-oxo-2,2,6,6-tetramethylpiperidine-1-oxyl(4-oxo-TEMPO) [Sigma Aldrich]. [1-13C] sodium acetate, glycerol, and deuterated d8-glycerol were purchased from Sigma Aldrich. ProHance Gd3+ contrast agent was purchased from Bracco Diagnostics, New Jersey as 0.5 M Gd–HP–DO3A solution in water. Deuterium used as a solvent in this study was obtained from Cambridge Isotope Laboratories (Tewksbury, Massachusetts). The samples are prepared by mixing the materials following a procedure described below and used without further purification.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v glycerol/water glassing solvent was prepared and doped with trityl OX063 (15 mM) as a reference sample. For the deuterated sample, 1
1 v/v glycerol/water glassing solvent was prepared and doped with trityl OX063 (15 mM) as a reference sample. For the deuterated sample, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v d8-glycerol/D2O was used in place of the glycerol/water glassing solvent. The sample doped with Gd3+ was prepared by adding a trace amount of ProHance contrast agent (2 mM). To ensure homogeneity, each sample was mixed using a vortex mixer for at least 3 minutes.
1 v/v d8-glycerol/D2O was used in place of the glycerol/water glassing solvent. The sample doped with Gd3+ was prepared by adding a trace amount of ProHance contrast agent (2 mM). To ensure homogeneity, each sample was mixed using a vortex mixer for at least 3 minutes.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v glycerol/water glassing solvent was prepared and doped with 4-oxo-TEMPO (40 mM) as a reference sample. For the deuterated sample, 1
1 v/v glycerol/water glassing solvent was prepared and doped with 4-oxo-TEMPO (40 mM) as a reference sample. For the deuterated sample, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v d8-glycerol/D2O was used in place of the glycerol/water glassing solvent. The sample doped with Gd3+ was prepared by adding a trace amount of ProHance contrast agent (2 mM). Each sample was mixed using a vortex mixer for at least 3 minutes.
1 v/v d8-glycerol/D2O was used in place of the glycerol/water glassing solvent. The sample doped with Gd3+ was prepared by adding a trace amount of ProHance contrast agent (2 mM). Each sample was mixed using a vortex mixer for at least 3 minutes.2.2 Dynamic nuclear polarization and data analysis
The samples were polarized in a homebuilt DNP system at the University of Florida utilizing an 89 mm room-temperature bore superconducting Bruker magnet energized to 5 T as described before.28 Samples were placed inside a cryostat and cooled down to T = 1.2 K. Due to the difficulty in obtaining the 13C thermal NMR signal at cryogenic temperatures, only the relative 13C polarization levels were plotted for each of the DNP samples. Relative 13C DNP polarization levels were used in previous studies to evaluate the effectiveness of DNP optimization methods especially in sample preparation practices.17,19,21 To ensure repeatability, all DNP measurements were done in triplicate. The mean values and standard deviations were calculated. The relative 13C NMR intensity was measured using a Bruker Avance III console (Bruker Biospin, MA) connected to a four-turn NMR saddle coil with a 24 mm height and 18 mm diameter surrounding the sample in the cryostat. For the polarization build up measurements, relative polarization level was initially zeroed by a series of RF pulses. Each sample was then polarized for up to 4 hours with continuous 50 mW microwave irradiation at a frequency of 140.71 GHz (4-oxo-TEMPO) or 140.31 GHz (trityl OX063) using a microwave source from Virginia Diodes, Inc. 13C NMR polarization build up was monitored every 200 s (4-oxo-TEMPO) or 300 s (trityl OX063) using a single, low-tip angle pulse (30 μs, 10 W). A signal acquisition time of 20 ms with 2048 data points was used followed by 1 kHz line broadening and Fast Fourier Transform (FFT). A linear baseline correction and zeroth order phase correction were applied to the Fourier transformed data. NMR signal intensity was measured by absolute integration. For determination of the optimal DNP microwave frequencies, similar signal acquisition and processing parameters were used, except polarization was monitored every 60 s for up to 12 minutes at each microwave frequency step. The DNP NMR data were plotted and analyzed using Igor Pro version 6.1 (Wavemetrics, OR).3. Results and discussion
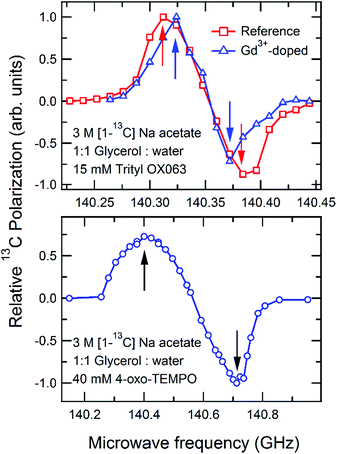
The results of the microwave frequency sweeps for reference samples doped with either 4-oxo-TEMPO or trityl OX063 radical are shown in Fig. 2. The optimum microwave frequencies for DNP are indicated by arrows, with both positive (P+) and negative (P−) maximum polarizations observed as expected. In trityl-doped samples, the addition of trace Gd3+ shifts the positive and negative maxima by approximately 12 MHz toward the center.Fig. 3 and 4 show the relative 13C polarization level of each of the trityl- and TEMPO-doped samples, normalized with respect to the reference samples which were set to unity. The data indicate that glassing solvent deuteration leads to a significant decrease, about 60%, in relative DNP-enhanced 13C polarization in the case of trityl-containing sample. On the other hand, this sample preparation method leads to about 40% improvement in the relative 13C polarization for the sample doped with TEMPO. These behavior with glassing solvent deuteration can be attributed to the differences in the nuclear Zeeman heat load, depending upon which type of free radical is used for DNP.15,17
 | ||
| Fig. 4 The maximum polarization of the reference, deuterated, and Gd3+-doped samples containing trityl (a) or TEMPO (b) free radicals. | ||
As can be inferred from the structure, the nitroxide-based free radical 4-oxo-TEMPO has a large g-anisotropy and hyperfine coupling contribution from the surrounding nuclei, thus a relatively broad ESR linewidth of about 465 MHz at 5% base was observed at 100 K using W-band ESR.17 The ESR linewidth of TEMPO is larger than the Larmor frequencies of both protons (γ = 42.58 MHz T−1) and 13C (γ = 10.7 MHz T−1) spins even at 5 T. Therefore, in the TEMPO-doped samples, the DNP process is expected to proceed predominantly via the thermal mixing process where both 1H and 13C spins are simultaneously polarized.29 In thermal mixing, the nuclear Zeeman system (NZS) is being cooled down via thermal contact with the microwave-cooled electron dipolar system (EDS), with both systems eventually acquiring the same spin temperature.15,30–33 The effectiveness of the DNP cooling process is affected by the heat capacity of each system. The specific heat capacity of NZS, CZ, is proportional to ωI2, where ωI is the Larmor frequencies of the NMR-active nuclei present in the DNP sample.15 Substitution of protons with deuterons (2H γ = 6.54 MHz T−1) in the DNP sample via deuteration of the glassing matrix lowers the specific heat capacity of NZS due to the lower γ or Larmor frequency of 2H spins. Thermodynamically speaking, lower specific heat capacity of the deuterated sample means that the EDS can easily cool down the NZS, thus resulting in a lower nuclear spin temperature or a larger nuclear spin polarization.17 Thus, glassing solvent deuteration is still recommended for 13C DNP at 5 T when wide ESR linewidth free radical such as 4-oxo-TEMPO is used.
On the other hand, the case for the DNP with trityl OX063 is different. The carbon-centered free radical trityl OX063 has a narrow ESR spectrum with 5% base linewidth of 115 MHz at W-band due to its highly symmetrical structure and its free radical center is surrounded by mainly spin-less nuclei.17 The ESR linewidth of trityl OX063 is comparable to the 13C Larmor frequency so thermal mixing is the expected predominant DNP mechanism for 13C and other low-γ nuclei using trityl. It has been shown in previous studies3,5,18 that indeed trityl is more favorable for use in DNP of samples having low-γ spins such as 13C. On the other hand, the Larmor frequencies of high-γ nuclei such as protons are much larger than the trityl ESR linewidth, thus 1H DNP with trityl is expected to proceed mainly via solid effect.34 This also means that, when trityl OX063 is used, the proton Zeeman system is essentially decoupled from EDS, thus EDS only has to cool down the 13C spins. However, substitution of protons by deuterons in the glassing matrix leads to an additional heat load for the EDS.17,35 The 2H Larmor frequency is less than or comparable to the trityl ESR D, thus the 2H NZS is now thermally coupled to the trityl EDS. Microwave frequency sweep of 2H-enriched compound doped with trityl revealed that its positive and negative polarization peaks almost overlap with the optimum microwave frequencies of trityl-doped 13C samples at 3.35 T and 1.4 K as reported in a previous study.8 This implies that EDS now has to cool down not only the 13C spins but also the 2H spins, eventually leading to slightly higher spin temperature or relatively lower 13C DNP signal as shown in Fig. 3 and 4.15,17 Therefore, glassing solvent deuteration is not beneficial for 13C DNP at 5 T when trityl OX063 is used.
In the trityl-doped sample, it has to be emphasized that the addition of Gd3+ results in a significant shift in the optimum microwave frequency for polarization transfer.18 Therefore, the frequency for the microwave irradiation must be retuned when Gd3+ is used. The addition of 2 mM Gd3+ in the trityl sample gives merely a modest enhancement of 30% in the polarization at a field of 5 T, in contrast to the 100–300% increase observed at B0 = 3.35 T using [1-13C] pyruvate as a target nuclei.18–21 This result suggests that the polarization enhancement by an addition of Gd3+ decreases as the field increases. Interestingly, in the TEMPO-doped samples, the inclusion of Gd3+ results in a tiny decrease in the polarization. Such decrease was not observed at 3.35 T, unless the Gd3+ concentration exceeds the optimum value.18 Both results indicate that the benefits of adding Gd3+ diminish at a higher field.
In order to explain the results of Gd3+ doping, we consider the theoretical maximum limit of thermal mixing process in DNP, which is given by16,18
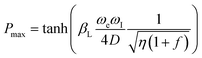 | (1) |
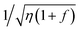 as the potential source of enhancement. The Gd3+ ions affect the polarization mainly by lowering the relaxation time for the electronic Zeeman system T1Z with no significant effect on the relaxation times of the nuclei.18 Therefore, this suggests that the polarization enhancement due to Gd3+ doping is a result of decreasing η. As mentioned before, the improvement in 13C DNP due to Gd-doping for trityl-doped samples is less pronounced at 5 T compared to the DNP optimization results observed at 3.35 T.18–21 This behavior may be ascribed to the fact that the trityl electron T1 are already shorter at higher magnetic fields,27 thus it is likely the extent of electron T1 reduction by Gd doping at higher fields is also less pronounced. Further ESR studies may be needed to elucidate this effect at higher magnetic fields. Nevertheless, Gd3+ doping is still advantageous for 13C DNP at 5 T using trityl OX063 as the polarizing agent.
as the potential source of enhancement. The Gd3+ ions affect the polarization mainly by lowering the relaxation time for the electronic Zeeman system T1Z with no significant effect on the relaxation times of the nuclei.18 Therefore, this suggests that the polarization enhancement due to Gd3+ doping is a result of decreasing η. As mentioned before, the improvement in 13C DNP due to Gd-doping for trityl-doped samples is less pronounced at 5 T compared to the DNP optimization results observed at 3.35 T.18–21 This behavior may be ascribed to the fact that the trityl electron T1 are already shorter at higher magnetic fields,27 thus it is likely the extent of electron T1 reduction by Gd doping at higher fields is also less pronounced. Further ESR studies may be needed to elucidate this effect at higher magnetic fields. Nevertheless, Gd3+ doping is still advantageous for 13C DNP at 5 T using trityl OX063 as the polarizing agent.
4. Conclusion
In conclusion, it has been shown that at B0 = 5 T, glassing solvent deuteration is still beneficial for 13C DNP when large ESR linewidth free radical such as 4-oxo-TEMPO is used. This effect results from the lowering of the heat capacity of the nuclear Zeeman system upon substitution of 1H with 2H spins, thus lowering the heat load for cooling by the electron dipolar system. Deuteration of the glassing matrix, however, is not recommended for 13C DNP using trityl OX063 at 5 T. Deuteration in the case of trityl OX063 leads to a larger heat load to the nuclear Zeeman system, by creating an additional thermal link between the 2H nuclear Zeeman system of the solvent and the electronic dipolar system, so 13C polarization decreases. In addition, inclusion of trace amounts of Gd3+ shifts the optimum microwave frequency for 13C DNP, so care must be taken when performing these experiments. A very slight decrease in 13C DNP signal were observed when Gd3+ is added to TEMPO-doped samples. On the other hand, Gd3+ doping is still recommended for 13C DNP at 5 T when trityl OX063 is used as the polarizing agent. The improvement, however, is relatively modest compared to the results at 3.35 T and this may be attributed to changes in electron relaxation behavior at higher fields. The optimization data obtained in this work may be useful in DNP sample preparation at higher fields, especially with the advent of the commercial clinical hyperpolarizer SPINlab™ which operates at a field of 5 T.Acknowledgements
The authors would like to thank these funding agencies for the financial support of this research: the U.S. Department of Defense grant award No. W81XWH-14-1-0048 and the Robert A. Welch Foundation grant No. AT-1877. Experiments were performed at the National High Magnetic Field Laboratory (NHMFL) which is supported by the National Science Foundation Cooperative Agreement No. DMR 1157490 and the State of Florida.Notes and references
- A. Abragam, The principles of nuclear magnetism, Oxford University Press, Oxford, 2004 Search PubMed
.
- J. H. Ardenkjær-Larsen, B. Fridlund, A. Gram, G. Hansson, L. Hansson, M. H. Lerche, R. Servin, M. Thaning and K. Golman, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 10158–10163 CrossRef PubMed
.
- L. L. Lumata, R. Martin, A. K. Jindal, Z. Kovacs, M. S. Conradi and M. E. Merritt, Magn. Reson. Mater. Phys., Biol. Med., 2014, 28, 195–205 CrossRef PubMed
.
- W. Jiang, L. Lumata, W. Chen, S. Zhang, Z. Kovacs, A. D. Sherry and C. Khemtong, Sci. Rep., 2015, 5, 9104 CrossRef PubMed
.
- L. Lumata, A. K. Jindal, M. E. Merritt, C. R. Malloy, A. D. Sherry and Z. Kovacs, J. Am. Chem. Soc., 2011, 133, 8673–8680 CrossRef CAS PubMed
.
- L. Lumata, M. E. Merritt, C. Malloy, A. D. Sherry and Z. Kovacs, Appl. Magn. Reson., 2012, 43, 69–79 CrossRef CAS
.
- L. Lumata, M. E. Merritt, Z. Hashami, S. J. Ratnakar and Z. Kovacs, Angew. Chem., Int. Ed., 2012, 51, 525–527 CrossRef CAS PubMed
.
- S. Reynolds and H. Patel, Appl. Magn. Reson., 2008, 34, 495–508 CrossRef CAS
.
- L. Lumata, C. Yang, M. Ragavan, N. Carpenter, R. J. DeBerardinis and M. E. Merritt, Methods Enzymol., 2015, 561, 73–106 Search PubMed
.
- A. Comment and M. E. Merritt, Biochemistry, 2014, 53, 7333–7357 CrossRef CAS PubMed
.
- J. Kurhanewicz, D. B. Vigneron, K. Brindle, E. Y. Chekmenev, A. Comment, C. H. Cunningham, R. J. DeBerardinis, G. G. Green, M. O. Leach, S. S. Rajan, R. R. Rizi, B. D. Ross, W. S. Warren and C. R. Malloy, Neoplasia, 2011, 13, 81–97 CrossRef CAS PubMed
.
- K. Golman and J. S. Petersson, Acad. Radiol., 2006, 13, 932–942 CrossRef PubMed
.
- K. M. Brindle, S. E. Bohndiek, F. A. Gallagher and M. I. Kettunen, Magn. Reson. Med., 2011, 66, 505–519 CrossRef PubMed
.
- S. J. Nelson, D. Vigneron, J. Kurhanewicz, A. Chen, R. Bok and R. Hurd, Appl. Magn. Reson., 2008, 34, 533–544 CrossRef CAS PubMed
.
- S. T. Goertz, Nucl. Instrum. Methods Phys. Res., Sect. A, 2004, 526, 28–42 CrossRef CAS
.
- J. Heckmann, W. Meyer, E. Radtke, G. Reicherz and S. Goertz, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 134418 CrossRef
.
- L. Lumata, M. E. Merritt and Z. Kovacs, Phys. Chem. Chem. Phys., 2013, 15, 7032–7035 RSC
.
- L. Lumata, M. E. Merritt, C. R. Malloy, A. D. Sherry and Z. Kovacs, J. Phys. Chem. A, 2012, 116, 5129–5138 CrossRef CAS PubMed
.
- J. W. Gordon, S. B. Fain and I. J. Rowland, Magn. Reson. Med., 2012, 68, 1949–1954 CrossRef CAS PubMed
.
- A. Flori, M. Liserani, S. Bowen, J. H. Ardenkjaer-Larsen and L. Menichetti, J. Phys. Chem. A, 2015, 119, 1885–1893 CrossRef CAS PubMed
.
- L. F. Waldner, A. Chen, W. Mander, T. Scholl and C. McKenzie, J. Magn. Reson., 2012, 223, 85–89 CrossRef PubMed
.
- J. H. Ardenkjaer-Larsen, S. Macholl and H. Jóhannesson, Appl. Magn. Reson., 2008, 34, 509–522 CrossRef CAS
.
- H. Jóhannesson, S. Macholl and J. H. Ardenkjaer-Larsen, J. Magn. Reson., 2009, 197, 167–175 CrossRef PubMed
.
- S. Jannin, A. Comment, F. Kurdzesau, J. A. Konter, P. Hautle, B. van den Brandt and J. J. van der Klink, J. Chem. Phys., 2008, 128, 241102 CrossRef CAS PubMed
.
- S. Jannin, A. Bornet, R. Melzi and G. Bodenhausen, Chem. Phys. Lett., 2012, 549, 99–102 CrossRef CAS
.
- J. H. Ardenkjaer-Larsen, J. Magn. Reson., 2016, 264, 3–12 CrossRef CAS PubMed
.
- L. Lumata, Z. Kovacs, A. D. Sherry, C. Malloy, S. Hill, J. van Tol, L. Yu, L. Song and M. E. Merritt, Phys. Chem. Chem. Phys., 2013, 15, 9800–9807 RSC
.
- B. Lama, J. H. P. Collins, D. Downes, A. N. Smith and J. R. Long, NMR Biomed., 2016, 29, 226–231 CrossRef CAS PubMed
.
- A. Comment, B. van der Brandt, K. Uffman, F. Kurdzesau, S. Jannin, J. A. Konter, P. Hautle, W. T. Wenkebach, R. Gruetter and J. J. van der Klink, Concepts Magn. Reson., Part B, 2007, 31, 255–269 CrossRef
.
- M. Borghini and K. Scheffler, Phys. Rev. Lett., 1971, 26, 1362–1365 CrossRef CAS
.
- M. Borghini, W. de Boer and K. Morimoto, Phys. Lett. A, 1974, 48, 244–246 CrossRef
.
- M. Borghini, Phys. Rev. Lett., 1968, 20, 419–421 CrossRef CAS
.
- W. de Boer, M. Borghini, K. Morimoto, T. O. Niinikoski and F. J. Udo, J. Low Temp. Phys., 1974, 15, 249 CrossRef CAS
.
- J. Wolber, F. Ellner, B. Fridlund, A. Gram, H. Johannesson, G. Hansson, L. H. Hansson, M. H. Lerche, S. Mansson, R. Servin, M. Thaning, K. Golman and J. H. Ardenkjaer-Larsen, Nucl. Instrum. Methods Phys. Res., Sect. A, 2004, 526, 173–181 CrossRef CAS
.
- F. Kurdzesau, B. van der Brandt, A. Comment, P. Hautle, S. Jannin, J. J. van der Klink and J. A. Konter, J. Phys. D: Appl. Phys., 2008, 41, 155506 CrossRef
.
Footnote |
| † These authors provided equal contribution. |
| This journal is © The Royal Society of Chemistry 2016 |