Synthesis and performance of cross-linked PEDOT:MOI-P(SS-HEA) transparent conductive films by UV irradiation†
Lei Cao‡
,
Qianqiu Tang‡ and
Gengchao Wang*
Shanghai Key Laboratory of Advanced Polymeric Materials, Key Laboratory for Ultrafine Materials of Ministry of Education, School of Materials Science and Engineering, East China University of Science and Technology, Shanghai 200237, P.R. China. E-mail: gengchaow@ecust.edu.cn
First published on 16th March 2016
Abstract
Poly(3,4-ethylenedioxythiophene) (PEDOT) is a promising conductive polymer due to its high conductivity and optical transparency. However, two main challenges remain to be solved for its practical application, including improving the humidity stability and water resistance. Here, a novel cross-linkable copolymer of methacryloyloxyethyl isocyanate grafted poly(styrene sulfonate-co-2-hydroxyethyl acrylate) [MOI-P(SS-HEA)] is synthesized by a radical polymerization and condensation reaction. MOI-P(SS-HEA) is used as a multi-functional counter anion to obtain the UV-curable conductive dispersion of PEDOT:MOI-P(SS-HEA). The electrical conductivity, humidity stability, and water resistance of the cross-linked PEDOT:MOI-P(SS-HEA) [PEDOT:MOI-P(SS-HEA)-C] conductive films are investigated. The results indicate that the UV cross-linked reaction significantly improves the humidity stability and water resistance of the PEDOT:MOI-P(SS-HEA)-C conductive film.
1. Introduction
Intrinsic conducting polymers (ICP) have been attracting particular attention due to their plentiful application in many areas including capacitors,1 photovoltaics,2,3 electrochromic devices,4 polymer light-emitting diodes (PLEDs),5 antistatic coatings,6 and so on.7–11 Among various ICPs, poly(3,4-ethylenedioxythiophene) (PEDOT):poly(styrenesulfonate) (PSS) is one of the most promising organic-based electric materials with growing applications,12–18 due to its superior mechanical processability, excellent thermal stability, high conductivity and transparency. By introducing the water-soluble polyanion–PSS,19 the mechanical processability of PEDOT is enhanced. Whereas PSS also leads to the poor water resistance of PEDOT:PSS films. Furthermore, the conductivity of PEDOT:PSS films decreases in humidity,20 limiting the performance of the final devices such as organic photovoltaics.20–22 There are several reports attempted to increase the water resistance and humidity stability of PEDOT:PSS films.23–26 And chemical cross-linking provides the most effective approach by introducing chemical bonding between molecules. The cross-linking methods can generally be divided into two types based on the cross-link bonds. The first way is ionic cross-linking.27 M. Döbbelin, et al.28 improved water resistance of PEDOT:PSS films by replacing the cations associated with the sulfonate polyanion by large organic cations. G. Winroth, et al.29 cross-linked PEDOT:PSS by addition of a water-soluble ionic bis(fluorinated phenyl azide), with a view of increasing water resistance and using it as a hole-injector layer in light-emitting diodes. The other method is covalent cross-linking.30 Yin et al.31 synthesized poly(styrene sulfonate-co-N-methylol acrylamide):poly(3,4-ethylenedioxythiophene) composites by self cross-linking at different temperature, improving the mechanical property and weather stability compared with commercial PEDOT:PSS. Rodríguez et al.32 reported PEDOT:PSS films cross-linking with glycerol between 100 °C and 200 °C, which offered an impressive protection of a PEDOT:PSS-based layer against water. And Huang et al.33 studied the chemical cross-linking of PEDOT:PSS using poly(ethylene oxide) at 130 °C. Though thermal cross-linking does not need special devices, curing process under high temperature may cause the layer to shrink, crack and void. Another method of UV cross-linking has its own superiority, including rapid curing, nearly unlimited curing area and no affecting other components in the assembly. Thus, cross-linking using UV method is worth researching so as to improve the performance of PEDOT:PSS-based films.Herein, in order to improve the water resistance and humidity stability of PEDOT:PSS-based films, we designed a novel copolymer of methacryloyloxyethyl isocyanate grafting poly(styrene sulfonate-co-2-hydroxyethyl acrylate) [MOI-P(SS-HEA)] to replace commonly used PSS. The active double bond of 2-hydroxyethyl acrylate (HEA) allows it to copolymerize with sodium p-styrenesulfonate (SSNa) to prepare water dispersible copolymer, and the hydroxyl group can react with isocyanate of MOI to introduce active double bond for further UV cross-linking. Using the as-prepared MOI-P(SS-HEA) as a multi-functional counteranion for PEDOT, the obtained UV cross-linked PEDOT:MOI-P(SS-HEA) conductive film [PEDOT:MOI-P(SS-HEA)-C] exhibited excellent enhanced humidity stability and water resistance compared to the PEDOT:PSS.
2. Experimental
2.1 Synthesis of the P(SS-HEA) copolymers
A series of poly(styrene sulfonate-2-hydroxyethyl acrylate) [P(SS-HEA)] copolymers were synthesized by the solution polymerization as shown in Scheme 1. The sodium p-styrenesulfonate (SSNa, Aladdin) and 2-hydroxyethyl acrylate (HEA, Aladdin) were dissolved in deionized water. Then ammonium persulfate (APS, Shanghai Chemical Reagent Co.) aqueous solution was slowly added dropwise to the above solution. The polymerization continued at 75 °C for 12 h under the argon atmosphere. The products were precipitated in acetone, and then washed with acetone. After that, the precipitates were dried at 60 °C under vacuum. For comparison, poly(2-hydroxyethyl acrylate) (PHEA) and PSS homopolymers were synthesized through the similar procedure as above. The exact amounts of reagents were listed in Table 1.| Samples | R1a | SSNa (mmol) | HEA (mmol) | APS (mmol) | Deionized water (g) | R2b |
|---|---|---|---|---|---|---|
| a R1 is feed molar ratio of SSNa/HEA.b R2 is SSNa/HEA molar ratio in copolymers from 1H NMR analysis. | ||||||
| PSS | — | 90 | 0 | 1.8 | 60 | — |
| P(SS-HEA)-3 | 3/1 | 67.5 | 22.5 | 1.8 | 60 | 2.92/1 |
| P(SS-HEA)-4 | 4/1 | 72 | 18 | 1.8 | 60 | 3.74/1 |
| P(SS-HEA)-5 | 5/1 | 75 | 15 | 1.8 | 60 | 4.83/1 |
| P(SS-HEA)-6 | 6/1 | 77.1 | 12.9 | 1.8 | 60 | 5.77/1 |
| PHEA | — | 0 | 90 | 1.8 | 60 | — |
2.2 Synthesis of the MOI-P(SS-HEA)
10 g P(SS-HEA)-4 was dissolved in 60 mL DMSO (redistilled under vacuum and dried with molecular sieves before use) under argon atmosphere. And 29.9 mg catalyst dibutyltin dilaurate (DBTDL, Sigma-Aldrich) was added under stirring. Then methacryloyloxyethyl isocyanate (MOI, Aladdin) (molar ratio of MOI/HEA units = 2) was added in the above solution and the reaction was kept at 30 °C for 10 h (the completion of the reaction was monitored by FTIR with the disappearance of the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups) to obtain MOI-P(SS-HEA) (as shown in Scheme 1). The crude product was purified by precipitation in CH2Cl2, and then washed by CH2Cl2 and dried at 45 °C under vacuum for further use.
O groups) to obtain MOI-P(SS-HEA) (as shown in Scheme 1). The crude product was purified by precipitation in CH2Cl2, and then washed by CH2Cl2 and dried at 45 °C under vacuum for further use.
2.3 Synthesis of the PEDOT:MOI-P(SS-HEA) conductive dispersions
The conductive dispersions of PEDOT:MOI-P(SS-HEA) were synthesized by using the MOI-P(SS-HEA) as a counteranion. The feed molar ratio of SO3−/EDOT = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 [PEDOT:MOI-P(SS-HEA)]. 1.03 g 3,4-ethylenedioxythiophene (EDOT, Aladdin), 2.72 g MOI-P(SS-HEA), and Fe2(SO4)3·5H2O (7.2 mg) were dissolved in 100 mL deionized water. Then 0.04 g HCl (35.5 wt%) was added to keep pH = 1. After that, 4 g APS in 40 mL deionized water was dropwise in the above solution. The reaction was carried out at room temperature for 24 h under an argon atmosphere. The resulting PEDOT:MOI-P(SS-HEA) conductive dispersions were purified by dialysis with deionized water for three days, and the water was renewed every 12 h. The as-used dialysis membrane (Mw cut off = 3500) was purchased from Union Carbide.
1 [PEDOT:MOI-P(SS-HEA)]. 1.03 g 3,4-ethylenedioxythiophene (EDOT, Aladdin), 2.72 g MOI-P(SS-HEA), and Fe2(SO4)3·5H2O (7.2 mg) were dissolved in 100 mL deionized water. Then 0.04 g HCl (35.5 wt%) was added to keep pH = 1. After that, 4 g APS in 40 mL deionized water was dropwise in the above solution. The reaction was carried out at room temperature for 24 h under an argon atmosphere. The resulting PEDOT:MOI-P(SS-HEA) conductive dispersions were purified by dialysis with deionized water for three days, and the water was renewed every 12 h. The as-used dialysis membrane (Mw cut off = 3500) was purchased from Union Carbide.
For comparison, the corresponding PEDOT:PSS conductive dispersion was obtained by using the pure PSS, the feed molar ratio of SO3−/EDOT = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the other preparation condition was the same as PEDOT:MOI-P(SS-HEA).
1 and the other preparation condition was the same as PEDOT:MOI-P(SS-HEA).
2.4 Preparation of cross-linkable PEDOT:MOI-P(SS-HEA) conductive dispersions
For UV cross-linking of PEDOT:MOI-P(SS-HEA), the UV photosensitive agent (Irgacure 819DW, Ciba Specialty Chemicals Inc.) (6 wt% by MOI-P(SS-HEA)) was added into the obtained PEDOT:MOI-P(SS-HEA) conductive dispersions and the mixtures were stirred vigorously for 60 min before use.2.5 Preparation of cross-linked PEDOT:MOI-P(SS-HEA) conductive films
The obtained cross-linkable PEDOT:MOI-P(SS-HEA) dispersion were secondly doped via adding 5 wt% of DMSO in the dispersion, and then being magnetic stirred for 24 h. Then cross-linked PEDOT:MOI-P(SS-HEA) [PEDOT:MOI-P(SS-HEA)-C] conductive films (with or without 5 wt% of DMSO) were formed on glass substrates by spin-coating at 1000 rpm and irradiated with UV lamp (wavelength at 365 nm) for 45 s, and then dried under 70 °C. The thickness of the films was estimated by observing their cross-sectional view on cross-sectional field-emission scanning electron microscopy (FE-SEM) (Hitachi S-4800) (shown in Fig. S1†). In comparison, the PEDOT:MOI-P(SS-HEA) and PEDOT:PSS conductive films (with or without 5 wt% of DMSO doping) were formed on glass substrates in the same condition without UV irradiation. The above films were used to test electrical conductivity, water contact angles and water resistance.For humidity absorptivity testing, the PEDOT:MOI-P(SS-HEA)-C film was obtained by drop-casting the dispersions on tetrafluoroethylene substrate, irradiating with UV lamp for 180 s, and then drying under room temperature. In comparison, the PEDOT:MOI-P(SS-HEA) and PEDOT:PSS films were obtained in the same condition without UV irradiation.
2.6 Characterization
Fourier transform infrared (FTIR) spectroscopy was performed on a Nicolet 5700 spectrometer using KBr sample pellets. 1H-NMR spectra were recorded using a Bruker AVANCE 500 NMR spectrometer. The mechanical properties of the samples were measured on a Zwick–Roell testing system at a stretching speed of 5 mm min−1. The electrical conductivities were measured by a RTS-8 four-point probe apparatus. The morphology of the samples was characterized using Hitachi S-4800 field-emission scanning electron microscopy (FE-SEM). Water contact angles were obtained by a JC2000D3 of POWEREACH (Shanghai Zhongchen Digital Technology CO., Ltd).For the measurement of humidity absorptivity, the dry solid weight of the films (Wsolid) was obtained after being dried at 120 °C under vacuum for 12 h firstly. Then, the films were put in an oven at 25 °C with different relative humidity (RH) (30% RH, 60% RH and 100% RH) for 72 h. And after absorbing the humidity, the weight of humidity-absorbing films (Whumidity-absorbing) was measured. The humidity absorptivity was calculated following equation: humidity absorptivity (100%) = (Whumidity-absorbing − Wsolid)/Wsolid × 100%. In the water resistance test, the PEDOT:MOI-P(SS-HEA)-C and PEDOT:PSS conductive films were immersed in water with slow water flow (0.1 m3 min−1), and the photographs after testing were recorded using a digital camera (D7100, Nikon).
3. Results and discussion
The FTIR spectra of PSS and P(SS-HEA) copolymers with various SSNa/HEA molar ratios are shown in Fig. 1. The spectrum of PSS exhibited the main bands at 1601, 1250–1140, 1040, and 1009 cm−1, which were assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration in the benzenoid rings, the SO3− asymmetric stretching vibration and the SO3− symmetric stretching vibration,31 respectively. For the P(SS-HEA) copolymers, the characteristic bands attributed to the PSS were observed. Besides, a new band at 1727 cm−1 to the C
C stretching vibration in the benzenoid rings, the SO3− asymmetric stretching vibration and the SO3− symmetric stretching vibration,31 respectively. For the P(SS-HEA) copolymers, the characteristic bands attributed to the PSS were observed. Besides, a new band at 1727 cm−1 to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration in HEA units also appeared, confirming the successful copolymerization between SSNa and HEA. Furthermore, compared to band at 1601 cm−1, the intensity of the band at 1727 cm−1 increases with the decreasing SSNa/HEA molar ratios, indicating that the content of HEA in the copolymers raises with the increasing of the HEA feeding amounts.
O stretching vibration in HEA units also appeared, confirming the successful copolymerization between SSNa and HEA. Furthermore, compared to band at 1601 cm−1, the intensity of the band at 1727 cm−1 increases with the decreasing SSNa/HEA molar ratios, indicating that the content of HEA in the copolymers raises with the increasing of the HEA feeding amounts.
To determine the chemical composition, the 1H-NMR analyses were employed (Fig. 2). For the PSS, the two peaks at 7.5 (peak A) and 6.8 ppm (peak B) were ascribed to the two protons each of aromatic ring, and the peak at 1.4 ppm (peak C) was assigned to three protons on the main chain of PSS. The integration area of peak A, B, and C is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.94, which is close to the actual ratio (2
2.94, which is close to the actual ratio (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). In the P(SS-HEA) copolymers, the peak A at 7.5 ppm and peak B at 6.8 ppm are also assigned to the two protons each of aromatic ring, the peak E at 1.4 ppm is due to three protons on the main chain of SSNa unit, and the –CH2– on the main chain of HEA unit. Besides, the peak D at 3.2 ppm is related to the –CH– on the main chain of HEA unit and –OCH2CH2O– of HEA unit.34 The molar ratios of SSNa/HEA in the copolymers could be calculated from the formula of 7area(A + B)/4area(D + E − C) (Table 1). The above results show that the compositions of SSNa and HEA in copolymers are close to the feeding ratio.
3). In the P(SS-HEA) copolymers, the peak A at 7.5 ppm and peak B at 6.8 ppm are also assigned to the two protons each of aromatic ring, the peak E at 1.4 ppm is due to three protons on the main chain of SSNa unit, and the –CH2– on the main chain of HEA unit. Besides, the peak D at 3.2 ppm is related to the –CH– on the main chain of HEA unit and –OCH2CH2O– of HEA unit.34 The molar ratios of SSNa/HEA in the copolymers could be calculated from the formula of 7area(A + B)/4area(D + E − C) (Table 1). The above results show that the compositions of SSNa and HEA in copolymers are close to the feeding ratio.
It was interesting to explore the optimal ratio of SSNa/HEA to achieve the optimal P(SS-HEA) copolymer. The mechanical performance of the final conductive films is mainly determined by the P(SS-HEA) copolymers because PEDOT and its oligomers have negligible effect on the mechanical moduli of long PSS polymer chain.35–37 Besides, compared to PEDOT, PSS was the main content in the conductive film. Tensile modulus and strength for the P(SS-HEA) copolymers with various feeding ratios of SSNa/HEA were summarized in Fig. 3. Moreover, as shown in Fig. S2,† the introduction of HEA into the copolymers reduced the rigidity of main chain, resulting in improving the ability of film formation of P(SS-HEA) copolymers. Furthermore, for P(SS-HEA) copolymers, the tensile modulus slightly decreased with the molar ratios of SSNa/HEA increasing from 3/1 to 5/1, and then abruptly decreased (Fig. 3a). While the tensile strength first increased with the increasing molar ratios of SSNa/HEA from 3/1 to 4/1, and thereafter, it gradually decreased (Fig. 3b). At the SSNa/HEA molar ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, both the tensile modulus and tensile strength show the maximum values and are 1470 MPa and 34.9 MPa, respectively. In addition, the water solubility of the P(SS-HEA) decreased when the SSNa/HEA molar ratios are too low, and the P(SS-HEA) copolymers are insoluble in water when the molar ratios of SSNa/HEA are equal or lower than 2/1 (Fig. S3†). Considering both the mechanical performance and water-solubility, the optimal SSNa/HEA molar ratio of 4/1 is chosen for the following studies, and the copolymer is designated as P(SS-HEA)-4.
1, both the tensile modulus and tensile strength show the maximum values and are 1470 MPa and 34.9 MPa, respectively. In addition, the water solubility of the P(SS-HEA) decreased when the SSNa/HEA molar ratios are too low, and the P(SS-HEA) copolymers are insoluble in water when the molar ratios of SSNa/HEA are equal or lower than 2/1 (Fig. S3†). Considering both the mechanical performance and water-solubility, the optimal SSNa/HEA molar ratio of 4/1 is chosen for the following studies, and the copolymer is designated as P(SS-HEA)-4.
 | ||
| Fig. 3 (a) Tensile modulus and (b) tensile strength of P(SS-HEA) copolymers with various SSNa/HEA molar ratios. | ||
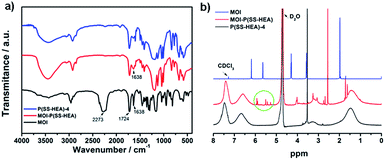
To endow curable characteristics of P(SS-HEA) copolymers, we introduced the double bonds through grafting methacryloyloxyethyl isocyanate (MOI) onto P(SS-HEA)-4. The chemical structure of MOI-P(SS-HEA) was characterized by FT-IR and 1H-NMR analysis. As shown in Fig. 4a, the MOI exhibited the main bands at 2273, 1724 and 1638 cm−1 corresponding to the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, the C
O stretching vibration, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, and the C
O stretching vibration, and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration, respectively. After the grafting reaction, the spectrum of the MOI-P(SS-HEA) exhibited a band at 1638 cm−1, indicating the existence of the C
C stretching vibration, respectively. After the grafting reaction, the spectrum of the MOI-P(SS-HEA) exhibited a band at 1638 cm−1, indicating the existence of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bands. Fig. 4b shows the 1H-NMR (D2O, CDCl3 as solvent) spectra of P(SS-HEA)-4, MOI-P(SS-HEA) and MOI. For the MOI, the two peaks at 5.6 and 6.2 ppm correspond to the two protons of the –C
C double bands. Fig. 4b shows the 1H-NMR (D2O, CDCl3 as solvent) spectra of P(SS-HEA)-4, MOI-P(SS-HEA) and MOI. For the MOI, the two peaks at 5.6 and 6.2 ppm correspond to the two protons of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, the peak at 2.0 ppm is related to protons on the –CH3 group, and the two peaks at 3.5 and 4.3 ppm are assigned to the two protons each of the –CH2CH2–. For the MOI-P(SS-HEA), several peaks near 5–6 ppm are clearly detected, which correspond to the C
CH2, the peak at 2.0 ppm is related to protons on the –CH3 group, and the two peaks at 3.5 and 4.3 ppm are assigned to the two protons each of the –CH2CH2–. For the MOI-P(SS-HEA), several peaks near 5–6 ppm are clearly detected, which correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond of MOI.
C double bond of MOI.
The morphology of the PEDOT:PSS and PEDOT:MOI-P(SS-HEA) conductive dispersions were investigated by the TEM analysis. As shown in Fig. 5, the average particle size of PEDOT:PSS is 174 nm, while that of PEDOT:MOI-P(SS-HEA) is 118 nm. This indicates that the introduction of the MOI-P(SS-HEA) counteranion improved the PEDOT dispersion compared to the PSS counteranion. The composition of PEDOT:MOI-P(SS-HEA) and PEDOT:PSS conductive films were determined by the XPS analyses. Fig. 6 shows the XPS spectra for PEDOT:PSS and PEDOT:MOI-P(SS-HEA) films. S(2p) peaks are observed in both films at the binding energy of 167.6 and 163.9 eV, which correspond to the sulfur signals from the sulfonate and thiophene of PSS (or MOI-P(SS-HEA)) and PEDOT, respectively.2 The sulfur molar ratios of PSS/PEDOT and MOI-P(SS-HEA)/PEDOT films are calculated from the relative area of S 2p peaks, which are 1.83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1.81
1 and 1.81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. Furthermore, the content of PEDOT in PEDOT:PSS and PEDOT:MOI-P(SS-HEA) films are calculated and are 27.36 wt% and 26.37 wt%, respectively. In a word, there is little influence on the content of the PEDOT (conductive component) by using the MOI-P(SS-HEA) as the counteranion. Thus, the conductivity of PEDOT:MOI-P(SS-HEA) and PEDOT:PSS films is approximative (Table 2).
1, respectively. Furthermore, the content of PEDOT in PEDOT:PSS and PEDOT:MOI-P(SS-HEA) films are calculated and are 27.36 wt% and 26.37 wt%, respectively. In a word, there is little influence on the content of the PEDOT (conductive component) by using the MOI-P(SS-HEA) as the counteranion. Thus, the conductivity of PEDOT:MOI-P(SS-HEA) and PEDOT:PSS films is approximative (Table 2).
| Film | Conductivity without DMSO (S cm−1) | Conductivity with 5 wt% DMSO (S cm−1) |
|---|---|---|
| a The conductivity was averaged by five pieces of the films with the thickness about 200 nm. | ||
| PEDOT:PSS | 0.13 | 3.21 |
| PEDOT:MOI-P(SS-HEA) | 0.12 | 1.27 |
| PEDOT:MOI-P(SS-HEA)-C | 0.07 | 0.97 |
To improve the conductivity, the obtained PEDOT:MOI-P(SS-HEA) and PEDOT:PSS were secondly doped via adding 5 wt% of DMSO. Table 2 shows the conductivity of the thin films. After second doping with 5 wt% DMSO, the conductivity of PEDOT:MOI-P(SS-HEA) films almost improved ten-folds, while PEDOT:PSS improved more than twenty-folds. The reason for conductivity enhancement by DMSO is the conformational change of the PEDOT chains.38,39 At the same time, in the SEM observations (shown in Fig. S4†), the PEDOT:PSS and PEDOT:MOI-P(SS-HEA) films showed small aggregations. Yet, after secondary-doping by 5 wt% DMSO, the small aggregations disappeared both in the PEDOT:PSS and PEDOT:MOI-P(SS-HEA) films.40,41
The effect of relative humidity (RH) on the humidity absorptivity of PEDOT:PSS, PEDOT:MOI-P(SS-HEA) and PEDOT:MOI-P(SS-HEA)-C films are shown in Fig. 7. In 30% RH, PEDOT:PSS, PEDOT:MOI-P(SS-HEA) and PEDOT:MOI-P(SS-HEA)-C films shows approximative humidity absorptivity (lower than 4%). In 60% RH, the humidity absorptivity of PEDOT:PSS film is 7.8% while PEDOT:MOI-P(SS-HEA) film shows 14.5%. In comparison, PEDOT:MOI-P(SS-HEA)-C film absorbed 3.9% humidity, which is only 1/2 and 1/3 of the PEDOT:PSS and PEDOT:MOI-P(SS-HEA) films. In addition, at 100% RH, the humidity absorptivity of PEDOT:PSS film exceeded 30%, and PEDOT:MOI-P(SS-HEA) film is about 40%, while PEDOT:MOI-P(SS-HEA)-C is lower than 15%. The higher humidity absorptivity of PEDOT:MOI-P(SS-HEA) may be due to the unreacted HEA segment in MOI-P(SS-HEA). In comparison, after cross-linking, the humidity stability was significantly enhanced, which was owing to that the cross-linking structure prevented the further penetration of the humidity. Fig. 8 shows the contact angles of the PEDOT:PSS and PEDOT:MOI-P(SS-HEA)-C conductive films. After dropping water on the films for 30 s, the contact angle of PEDOT:PSS is 53°, showing good water wettability. In comparison, the contact angle of the PEDOT:MOI-P(SS-HEA)-C is 78°. This enhancement of contact angle indicated that after chemical cross-linking, the hydrophily of PEDOT:MOI-P(SS-HEA)-C decreased. In addition, this phenomenon may contribute to the better water resistance of PEDOT:MOI-P(SS-HEA)-C conductive film discussed below.
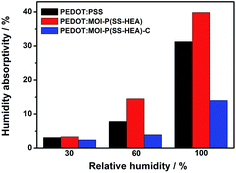 | ||
| Fig. 7 The Humidity absorptivity of PEDOT:PSS, PEDOT:MOI-P(SS-HEA) and PEDOT:MOI-P(SS-HEA)-C films at 25 °C for 72 h at 40% RH, 60% RH, and 100% RH. | ||
In the water resistance test, the PEDOT:PSS and PEDOT:MOI-P(SS-HEA)-C films were immersed in water with slow water flow (0.1 m3 min−1). The PEDOT:PSS film swelled in the water in the 1st min. After 10 min, large parts of the PEDOT:PSS film departed from the substrates and broke into pieces in the water (shown in Fig. 9a). On the contrary, the UV cross-linked PEDOT:MOI-P(SS-HEA)-C conductive film could remain almost the original shape on the substrate. And the swelling phenomenon only existed on the edge part (shown in Fig. 9b). This definitely confirmed that by introducing cross-linking segment MOI-HEA into PEDOT:PSS-based film, the cross-linking network in the film could improve the water resistance effectively.
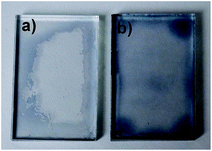 | ||
| Fig. 9 The photographs of the (a) PEDOT:PSS and (b) PEDOT:MOI-P(SS-HEA)-C films after water resistance test. | ||
4. Conclusions
In summary, we have designed a new multi-functional counteranion, methacryloyloxyethyl isocyanate grafting poly(styrenesulfonate-co-2-Hydroxyethyl acrylate) [MOI-P(SS-HEA)], for poly(3,4-ethylenedioxythiophene) (PEDOT), and fabricated a cross-linked structure in PEDOT:MOI-P(SS-HEA)-C films. By adding HEA segment into PSS backbones, the rigidity of PSS main chain is reduced. As a result, the filming ability of P(SS-HEA) is superior than PSS. The conductivity of the PEDOT:MOI-P(SS-HEA)-C film is in the same magnitude with PEDOT:PSS film. Whereas, PEDOT:MOI-P(SS-HEA)-C films shows higher humidity stability and water resistance than PEDOT:PSS films. And the contact angle of the PEDOT:MOI-P(SS-HEA)-C film was 78° in contrast to the 53° of PEDOT:PSS film, showing a decreasing hydrophily. The as-fabricated film with enhanced properties through UV cross-linking may have promising application in many areas such as transparent electric materials.Acknowledgements
We greatly appreciate the financial supports of National Natural Science Foundation of China (51173042), and Shanghai Municipality Research Project (15520720500).Notes and references
- H. Zhou, G. Han, Y. Chang, D. Fu and Y. Xiao, J. Power Sources, 2015, 274, 229–236 CrossRef CAS.
- Y. H. Kim, C. Sachse, M. L. Machala, C. May, L. Müller-Meskamp and K. Leo, Adv. Funct. Mater., 2011, 21, 1076–1081 CrossRef CAS.
- F.-C. Tang, J. Chang, F.-C. Wu, H.-L. Cheng, S. L.-C. Hsu, J.-S. Chen and W.-Y. Chou, J. Mater. Chem., 2012, 22, 22409 RSC.
- V. K. Thakur, G. Ding, J. Ma, P. S. Lee and X. Lu, Adv. Mater., 2012, 24, 4071–4096 CrossRef CAS PubMed.
- S. Nau, N. Schulte, S. Winkler, J. Frisch, A. Vollmer, N. Koch, S. Sax and E. J. List, Adv. Mater., 2013, 25, 4420–4424 CrossRef CAS PubMed.
- A. G. Sadekar, D. Mohite, S. Mulik, N. Chandrasekaran, C. Sotiriou-Leventis and N. Leventis, J. Mater. Chem., 2012, 22, 100–108 RSC.
- Y. Shi, L. Peng, Y. Ding, Y. Zhao and G. Yu, Chem. Soc. Rev., 2015, 44, 6684–6696 RSC.
- G. A. Snook, P. Kao and A. S. Best, J. Power Sources, 2011, 196, 1–12 CrossRef CAS.
- M. V. Fabretto, D. R. Evans, M. Mueller, K. Zuber, P. Hojati-Talemi, R. D. Short, G. G. Wallace and P. J. Murphy, Chem. Mater., 2012, 24, 3998–4003 CrossRef CAS.
- B. Lu, S. Zhen, S. Zhang, J. Xu and G. Zhao, Polym. Chem., 2014, 5, 4896 RSC.
- M. Chen, S. Duan, L. Zhang, Z. Wang and C. Li, Chem. Commun., 2015, 51, 3169–3172 RSC.
- R. Yue and J. Xu, Synth. Met., 2012, 162, 912–917 CrossRef CAS.
- B. Groenendaal, F. Jonas, D. Freitag, H. Pielartzik and J. Reynolds, Adv. Mater., 2000, 12, 481–494 CrossRef.
- S. Kirchmeyer and K. Reuter, J. Mater. Chem., 2005, 15, 2077 RSC.
- M. Vosgueritchian, D. J. Lipomi and Z. Bao, Adv. Funct. Mater., 2012, 22, 421–428 CrossRef CAS.
- Y. Zheng, D. Lee, H. Y. Koo and S. Maeng, Carbon, 2015, 81, 54–62 CrossRef CAS.
- M. Wagner, C. D. O'Connell, D. G. Harman, R. Sullivan, A. Ivaska, M. J. Higgins and G. G. Wallace, Synth. Met., 2013, 181, 64–71 CrossRef CAS.
- D. A. Mengistie, M. A. Ibrahem, P. C. Wang and C. W. Chu, ACS Appl. Mater. Interfaces, 2014, 6, 2292–2299 CAS.
- M. Lefebvre, Z. Qi, D. Rana and P. Pickup, Chem. Mater., 1999, 11, 262–268 CrossRef CAS.
- V. M. Drakonakis, A. Savva, M. Kokonou and S. A. Choulis, Sol. Energy Mater. Sol. Cells, 2014, 130, 544–550 CrossRef CAS.
- K. Norrman, M. V. Madsen, S. A. Gevorgyan and F. C. Krebs, J. Am. Chem. Soc., 2010, 132, 16883–16892 CrossRef CAS PubMed.
- K. Kawano, R. Pacios, D. Poplavskyy, J. Nelson, D. D. C. Bradley and J. R. Durrant, Sol. Energy Mater. Sol. Cells, 2006, 90, 3520–3530 CrossRef CAS.
- M. Wagner, G. Lisak, A. Ivaska and J. Bobacka, Sens. Actuators, B, 2013, 181, 694–701 CrossRef CAS.
- Z. Wang, J. Xu, Y. Yao, L. Zhang, Y. Wen, H. Song and D. Zhu, Sens. Actuators, B, 2014, 196, 357–369 CrossRef CAS.
- Z. Wang, Z. Wang, H. Zhang, X. Duan, J. Xu and Y. Wen, RSC Adv., 2015, 5, 12237–12247 RSC.
- H. Wang and V. Kumar, RSC Adv., 2015, 5, 9650–9657 RSC.
- S. R. Ghosh, J. Inganas and O. Inganas, Adv. Mater., 1998, 10, 1097–1099 CrossRef CAS.
- M. Döbbelin, R. Marcilla, C. Tollan, J. A. Pomposo, J.-R. Sarasua and D. Mecerreyes, J. Mater. Chem., 2008, 18, 5354 RSC.
- G. Winroth, G. Latini, D. Credgington, L.-Y. Wong, L.-L. Chua, P. K. H. Ho and F. Cacialli, Appl. Phys. Lett., 2008, 92, 103308 CrossRef.
- J. Y. Lee, Synth. Met., 2006, 156, 537–540 CrossRef CAS.
- H.-E. Yin, C.-F. Lee and W.-Y. Chiu, Polymer, 2011, 52, 5065–5074 CrossRef CAS.
- A. B. Rodríguez, M. M. Voigt, S. J. Martin, T. J. Whittle, R. M. Dalgliesh, R. L. Thompson, D. G. Lidzey and M. Geoghegan, J. Mater. Chem., 2011, 21, 19324 RSC.
- T.-M. Huang, S. Batra, J. Hu, T. Miyoshi and M. Cakmak, Polymer, 2013, 54, 6455–6462 CrossRef CAS.
- R. K. Bose and K. K. S. Lau, Chem. Vap. Deposition, 2009, 15, 150–155 CrossRef CAS.
- D. Tahk, H. H. Lee and D.-Y. Khang, Macromolecules, 2009, 42, 7079–7083 CrossRef CAS.
- U. Lang, N. Naujoks and J. Dual, Synth. Met., 2009, 159, 473–479 CrossRef CAS.
- S. Savagatrup, E. Chan, S. M. Renteria-Garcia, A. D. Printz, A. V. Zaretski, T. F. O'Connor, D. Rodriquez, E. Valle and D. J. Lipomi, Adv. Funct. Mater., 2015, 25, 427–436 CrossRef CAS.
- J. Ouyang, Q. Xu, C.-W. Chu, Y. Yang, G. Li and J. Shinar, Polymer, 2004, 45, 8443–8450 CrossRef CAS.
- Y. Xia and J. Ouyang, J. Mater. Chem., 2011, 21, 4927 RSC.
- U. Lang, E. Mueller, N. Naujoks and J. Dual, Adv. Funct. Mater., 2009, 19, 1215–1220 CrossRef CAS.
- A. M. Nardes, R. A. J. Janssen and M. Kemerink, Adv. Funct. Mater., 2008, 18, 865–871 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra02859d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |