High thermal stability and high tensile strength terpolyester nanofibers containing biobased monomer: fabrication and characterization†
Hoik Leea,
Jun Mo Koob,
Daewon Sohnc,
Ick-Soo Kim*a and
Seung Soon Im*b
aNano Fusion Technology Research Lab, Division of Frontier Fibers, Institute for Fiber Engineering (IFES), Interdisciplinary Cluster for Cutting Edge Research (ICCER), Shinshu University, 3-15-1, Tokida, Ueda, Nagano 386-8567, Japan
bDepartment of Organic and Nano Engineering, College of Engineering, Hanyang University, 17 Haengdang-dong, Seongdong-gu, Seoul, 133-791, Korea
cDepartment of Chemistry and Research Institute for Natural Sciences, Hanyang University, Seoul 133-791, Korea
First published on 12th April 2016
Abstract
This research fabricated novel nanofibers with a terpolyester of isosorbide, ethylene glycol, 1,4-cyclohexane dimethanol, and terephthalic acid (PEICT) using electrospinning and characterized their properties. The nanofibers have higher glass transition temperature (Tg) than other polyester-type polymers, and a smaller diameter nanofiber has higher Tg than a larger diameter nanofiber. This is due to the orientation of polymer chains inside nanofibers, which was verified by DSC and polarized ATR-FTIR. The morphology and diameter of the nanofibers affected by concentration of PEICT solution were studied by SEM. It demonstrated smooth and well-formed nanofibers, and showed an increase of the diameter with increasing concentration. In addition, the tensile property, which was confirmed by UTM, was enhanced with increasing diameter because molecular orientation existed in finer nanofibers. They show a better tensile property than general biobased nanofibers such as silk, chitosan, and gelatin. Finally, fabrication of PEICT nanofibers was optimized and characterized. They can be utilized in various industrial applications such as tissue engineering, wound dressings, and health care devices.
Introduction
Biobased polymers have attracted great interest in the polymer industry due to their eco-friendly property and providing positive environmental benefits.1 They have a lot of advantages such as energy recovery, CO2 reduction and biodegradability.2,3 They have offered researchers a possible opportunity to replace traditional petroleum-derived plastics and are used in various fields such as films, sheets, cosmetics, etc.4 However, they have relatively poor thermal and mechanical properties for engineering polymers.Isosorbide (1,4:3,6-dianhydrohexitol; ISB) is one of the most favorable candidates for renewable monomers used in addressing environmental issues.5,6 It is derived from glucose through dehydration of sorbitan. It has been used in various polymer preparations, pharmaceuticals, and cosmetics taking advantage of its two hydroxyl groups.7–11 Its resulting polymer shows high glass transition temperature (Tg) and transparency12 due to the uniqueness of its molecular structure and chirality. The use of ISB has been examined in polyesters,13 epoxies,14 polyurethanes,15 and polycarbonates.16,17 Polyester containing ISB is not only environmentally desirable, but also has superior thermal and optical properties due to the chirality of its asymmetrical hydroxyl groups and its molecular rigidity. Terpolyester containing ethylene glycol, 1,4-cyclohexane dimethanol, and terephthalic acid, along with biobased monomer ISB (PEICT; presented in Scheme 1), can be one candidate for an eco-friendly material which can contribute in part to delaying the depletion of petroleum and energy sources.
 | ||
| Scheme 1 Structure of PEICT. The chemical composition of EG (l), ISB (m), and CHDM (n) is 42.9, 7.5, and 48.5 molar ratio which is confirmed by NMR in a previous paper.18 | ||
We already have studied and reported the synthesis method and mechanism of PEICT.18,19 PEICT was synthesized using two-step melt polymerization due to its complicated reaction and the two hydroxyl groups in ISB monomer have different reactivity, and therefore ISB prevents the achievement of high-molecular-weight polyester.20 To get over this problem, 1,4-cyclohexane dimethanol (CHDM) was incorporated to assist the reaction between terephthalic acid (TPA) and ISB. Finally, we obtained a polyester containing ISB with Mn from 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 25
000 to 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and Tg from 90 to 140 °C. We also described the polycondensation mechanism for PEICT terpolyester and the effects of ethylene glycol (EG) as a chain linker in this polymer as a result of a topological selective chain scission process. We enhanced the mechanical and thermal properties, which is weak point of general biobased polymers, by utilizing additional monomer such as TPA, EG and CHDM.
000 and Tg from 90 to 140 °C. We also described the polycondensation mechanism for PEICT terpolyester and the effects of ethylene glycol (EG) as a chain linker in this polymer as a result of a topological selective chain scission process. We enhanced the mechanical and thermal properties, which is weak point of general biobased polymers, by utilizing additional monomer such as TPA, EG and CHDM.
The electrospinning technique is an attractive, simple and well-developed electro-hydrodynamic method used to produce various polymeric fiber sheets from nanometer to micrometer size in diameter with a large surface area to volume ratio.21,22 Numerous materials have been spun into nanofibers, by static electric forces, which possess a three-dimensional porous structure microscopically.23,24 Dissolved polymer forms a fiber structure during solvent evaporation in-flight which is deposited on the substrate. With this technique, a wide variety of polymers are fabricated25–27 and utilized in various ways such as membranes,28 wound healing,29 tissue engineering scaffolds,30,31 antibacterial materials,31,32 and others.33,34
In this paper, we fabricated novel nanofibers with PEICT using the electrospinning method to extend their application and investigated their properties. This kind of work has not been conducted yet and it will be significant for broadening the utilization of PEICT for industrial applications. Here, we optimized the fabrication condition of PEICT nanofiber, which has a high Tg compared to other biobased polymers, and confirmed its morphology and diameter by scanning electron microscopy (SEM). We used different concentrations of PEICT solution in the range of 8 wt% to 20 wt% for fabricating the nanofibers. As expected, a higher concentration led to a coarser diameter of PEICT nanofiber. The coarser PEICT nanofiber showed better mechanical properties in terms of tensile stress and strain. Especially, the nanofiber made with 20 wt% solution showed over 80% strain which is higher than that of other biobased polymers.35 Thermal property showed very intriguing results; the Tg peak shifts as the diameter of the nanofiber decreases. This phenomenon is due to an orientation of polymer chains inside a nanofiber that was confirmed by differential scanning calorimetry (DSC) and polarized attenuated total reflection Fourier transform infrared spectra (polarized ATR-FTIR).
Experimental
Materials
PEICT-T95 (ECOZEN T95) was kindly supplied by SK Chemicals, Republic of Korea. The pellets were used as received. The synthesis method of PEICT polyesters was demonstrated in our previous report.18 In our study, a sample with chemical composition of EG, ISB, and CHDM repeat units in 42.9, 7.5, and 48.5 molar ratio confirmed by NMR was used as presented in Scheme 1. Its weight-average molecular weight (Mw) is 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 g mol−1 determined by gel permeation chromatography. Chloroform (99%) and trifluoroacetic acid (98%) (TFA) were obtained from Wako and were used without any purification. PEICT solutions with different concentrations in chloroform/TFA were prepared with 3
400 g mol−1 determined by gel permeation chromatography. Chloroform (99%) and trifluoroacetic acid (98%) (TFA) were obtained from Wako and were used without any purification. PEICT solutions with different concentrations in chloroform/TFA were prepared with 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio. The range of mass concentrations was 8 wt% to 20 wt%. All solutions were stirred vigorously for 24 hours to fully dissolve the samples.
1 volume ratio. The range of mass concentrations was 8 wt% to 20 wt%. All solutions were stirred vigorously for 24 hours to fully dissolve the samples.
Electrospinning
In electrospinning, a high voltage is applied to a solution that rests on a sharp conducting tip. As a result of molecular ionization or charge redistribution, a Taylor cone is formed and a jet of the solution is extracted.36 The formed jet is then accelerated by the electric field and collected onto a substrate. An electrospinning apparatus equipped with a high-voltage power supply (Har-100*12, Matsusada Co., Tokyo, Japan), which is capable of generating voltages up to 100 kV, was used as the source of the electric field.37 A copper wire connected to a positive electrode (anode) was attached to an ejection needle with an inner diameter of 0.8 mm, and a negative electrode (cathode) was linked to a metallic drum (collector). A voltage of 15 kV was applied, the tip-to-collector distance was 25 cm, and flow rate of syringe pump was fixed at 0.4 ml h−1 in a room-temperature environment with ∼40% humidity for all electrospinning processes. To search for the optimum conditions, we changed the voltage from 6 kV to 20 kV and adjusted the distance from 10 cm to 30 cm. In addition, the flow rate was also varied from 0.3 ml h−1 to 0.5 ml h−1. From this trial and error, the conditions for electrospinning were optimized. Electrospinning was carried out carefully using concentrations of 8 wt%, 10 wt%, 12 wt%, 14 wt%, 16 wt%, 18 wt%, and 20 wt% as mentioned conditions.Characterization
The surface structure and morphology of PEICT nanofibers were studied via SEM (S-3000N by Hitachi, Japan) with an accelerating voltage of 15 kV. To confirm the quantity of nanofibers, all SEM samples were spun for the same time. Then a small piece was cut from each nanofiber mat made from different concentrations and then coated with Pt under a JEOL JFC-1200 fin coater for 60 s. The average diameter and distribution of nanofibers were measured from the SEM micrographs using image analysis software (Image J, version 1.49). To obtain a diameter distribution and its average value, fifty points in a single SEM image were randomly selected and evaluated in the diameter range of 100–1800 nm. The rheological properties of the PEICT solutions were determined with a rheometer (MARS III, Haake, Germany) using a 60 mm cone-and-plate geometry with a 1° cone angle at 25 °C. The shear viscosity was measured with a parallel plate geometry at a shear rate of 10 s−1.To evaluate the mechanical properties of the PEICT nanofiber mat, stress–train curves were obtained with a universal testing machine (UTM, OTT-003, Oriental TM, South Korea). Each PEICT solution had been spun for 1 day to make mat-type nanofibers. The thicknesses of PEICT mats were 0.06 mm (10 wt%), 0.09 mm (14 wt%) and 0.13 mm (20 wt%). The PEICT mats were prepared with a size of 15 mm (width) × 60 mm (length). The tensile tests were performed under standard conditions with a 3 kgf load cell at an extension rate of 10 mm min−1 and a gauge length of 30 mm.
DSC was performed to determine Tg of PEICT nanofibers. DSC curves were recorded with a TA-DSC 2010 instrument (TA instruments Q100) in the temperature range from 40 to 300 °C with a heating rate of 10 °C min−1 in N2 atmosphere. A pellet sample was melted at 290 °C for 5 min, and then quenched to 40 °C at a rate of 200 °C min−1 in N2 atmosphere. On the other hand, the PEICT nanofibers were scanned directly. All PEICT nanofiber samples were measured 3 times for obtaining more accurate results. Tg was estimated as the onset point in heat capacity associated with a transition.
The polarized ATR-FTIR spectra were recorded by a combination of a GladiATR spectrometer (PIKE Technologies) and Vertex 80v (Bruker). Aligned PEICT nanofibers produced using the negatively charged metal plates which have constant gap size (4 cm) were successfully obtained.38 To obtain orientation information, a set of two FTIR spectra was collected using two sample directions. During two sets of measurements of ATR-FTIR spectra, the sample was remounted at 90° sample rotation (two ATR-FTIR spectra; parallel (P‖) and perpendicular (P⊥)). The spectra were recorded from 400 to 4000 cm−1 with the coaddition of 32 scans. In parallel polarization, the direction of the oscillating electric field of the incident IR beam was parallel to the machine direction of the collection of the fibers (parallel to the fiber axis), while in perpendicular polarization it was perpendicular to the machine direction (perpendicular to the fiber axis). All absorbance values in the FTIR spectra were normalized to the absorbance of a reference peak at 1710 cm−1 that was not affected by the change in chain conformation and orientation.
Results and discussion
Morphology and trend of diameter
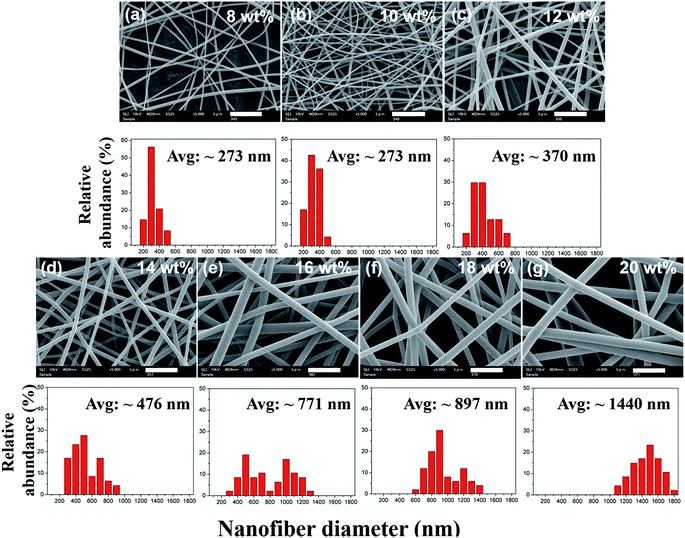
The surface morphologies of the PEICT nanofibers were evaluated using SEM (Fig. 1). It can be seen from the images that the PEICT nanofibers exhibit a randomly oriented, bead-free structure, and have smooth surfaces under the specified electrospinning conditions. As observed, the average diameters of nanofibers produced with different concentrations from 8 wt% to 20 wt% are different for each sample. In general, the nanofiber diameter could be altered with solution concentration. The fiber formation started at a concentration above 8 wt% of PEICT solution. It was likely to be sprayed rather than electrospun at a PEICT concentration lower than 8 wt%. As expected, the 8 wt% and 10 wt% concentrations resulted in the thinnest fiber diameters. However, the value for nanofibers from 8 wt% PEICT was less than that from 10 wt% PEICT when spun for the same time. By increasing the concentration, the solution showed increasing viscosity with densely entangled polymer chains (the relationship between viscosity and diameter is presented in Fig. S1†). Consequently, the diameter of PEICT nanofibers became thicker and had a wider distribution as the concentration increases (SEM image of each concentration is presented in Fig. 1).The diameters of PEICT nanofibers, made with 10 wt% concentration solution of PEICT, were highly populated within the range of 200–400 nm with an average diameter of 273 nm (Fig. 1(b)). This gradually increased to a larger diameter around 370 nm for the 12 wt% PEICT solution and started to broaden its distribution from this concentration. The diameter was around 456 nm for the 14 wt% PEICT solution and around 776 nm for the 16 wt% PEICT solution (Fig. 1(c–e)). Especially, the 16 wt% PEICT solution gave a wide distribution within the range of 600 nm to 1400 nm. At a concentration of 20 wt% of PEICT solution, the mean diameter was increased to around 1400 nm and retained its regularity slightly (Fig. 1(g)). The mean diameter of the PEICT nanofibers was fitted with a power law of PEICT concentration. This trend was similar to previous work performed on many other kinds of polymers.39,40
Mechanical properties of PEICT nanofiber mat
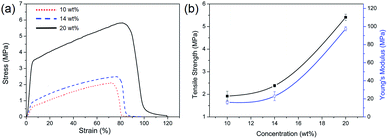
Tensile testing was conducted on all samples as types of mat. Fig. 2 shows representative stress–strain curves of the nanofibers fabricated with different concentrations. The elongation at break of several natural fibers such as cotton, hemp, flax, etc. show low tensile strain under 10%.1 In the case of silk, it has tensile strain under ∼30%35 and cellulose nanofiber, which is also a well-known biomaterial, has low tensile strain under 10%.41However, PEICT nanofibers showed a high tensile strain above 70%, as can be seen in Fig. 2(a). The mechanical properties of the PEICT nanofibers made with 10 wt% solution show the tensile strength as 1.9 MPa, and strain as 72.9%. In the case of 14 wt% and 20 wt% concentrations, the values are 2.3 MPa with 77% strain and 5.7 MPa with 81.8% strain, respectively. As the fiber diameter increases, an increase in strength and in Young's modulus was observed. The Young's modulus and tensile strength show a similar trend with increasing diameter. Both properties were also fitted with a power law of PEICT concentration, being the same as for diameter, as shown in Fig. 2(b). This points to the effect of diameter on tensile strength. In addition, the finest nanofibers showed the lowest strain at break. This could be explained by a higher degree of molecular orientation when finer fibers were produced in the electrical field, due to the increased fiber stretching.42 Wong et al. reported a higher molecular orientation affects the strength of nanofibers, and a similar trend was also presented by Hwang et al.43,44 Moreover, the uniqueness of the ISB structure, which is V-shaped with a 120° angle between the rings, assists in hindering the crystallization of the polymer chain.19 Therefore, the mechanical properties could be affected by the orientation of polymer chains.
Transition of thermal property of nanofibers
The chemical structure of ISB is unfavorable for crystallization, but it has a relatively high thermostability and low segmental mobility, and thus ISB units are interesting building blocks for polyesters having a high Tg. The highest Tg reported for aliphatic polyesters was 60 °C.45 We previously reported that the rigid structure and less flexible nature of ISB dramatically enhanced the positive effect of incorporating ISB on Tg.18 Tg of the PEICT is directly proportional to the ISB content, regardless of the quantity of EG and CHDM subunits in the polymer chain, and the estimated maximum Tg of the ISB homopolyester when the ISB content is 100% is about 195 °C.18Fig. 3 shows the second heating scans of the samples, carried out at 10 °C min−1 after the first heating scan with a 5 min isotherm to remove the thermal history. At this scan rate, none of the PEICT nanofibers showed an ability to crystallize, so they have the features of a completely amorphous polymer. Interestingly, all of nanofibers have a higher Tg than the pellet sample. The gap between pellet and nanofiber fabricated with 10 wt% concentration was around 6 °C (Tg of pellet was ∼95.5 °C, whereas that of nanofibers from 10 wt% solution was ∼101 °C). Furthermore, as mentioned above, the diameter of nanofibers is related to concentration. As presented in Fig. 3, the nanofibers fabricated with 20 wt% concentration, the diameter of which was around 1.4 μm, had the lowest Tg of the nanofiber samples of around 96.7 °C, and Tg increased slightly with decreasing diameter. That is, finer fibers have a higher glass transition temperature. This also could be explained by molecular chain orientation when nanofibers were produced.
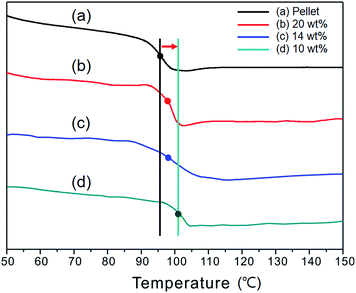 | ||
| Fig. 3 DSC curves of (a) pellet (black) and electrospun nanofibers with different concentrations: (b) 20 wt% (red), (c) 14 wt% (blue) and (d) 10 wt% (green). | ||
The molecular orientation was proved by polarized ATR-FTIR with aligned and randomly collected PEICT nanofibers, as presented in Fig. 4. In order to characterize the extent of orientation in uniaxially well-aligned fibers, the PEICT nanofibers were produced in the gap between negatively charged metal plates.
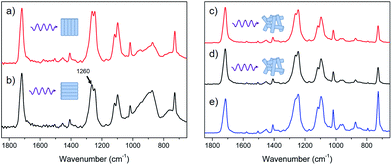
The assignments of the IR bands for the PEICT nanofibers were based on previously studied infrared data.46–48 The distinguishing difference in polarized ATR-FTIR is the peak at 1260 cm−1 which comes from the C–O stretch of ester group in PEICT.46 There are 3 kinds of C–O stretch such as EG–COO–terephthalate (TER), CHDM–COO–TER and ISB–COO–TER. Unfortunately, we did not determine the origin of C–O stretching exactly, but we confirmed obviously that there is molecular orientation in the nanofibers. In the case of the uniaxially well-aligned nanofibers, the intensity of the C–O stretch was relatively increased in the case of parallel polarization (Fig. 4(b)).
Assuming that the molecular chains were oriented along uniaxially well-aligned nanofiber axis, it can be expected that the ester group in PEICT should be parallel to the fiber axis. Therefore, the absorption of the incoming parallel polarized IR beam by the C–O stretching would be increased. On the other hand, when the incident IR beam is perpendicular to the fiber axis, the C–O stretching is not resonant with the electric field of the incoming polarized IR beam. Therefore, the C–O stretching absorption should become weak for the perpendicular polarization. In contrast, any kind of peak change for randomly collected nanofibers was not shown up in parallel and perpendicular directions, and it also showed the same spectrum as the PEICT pellet (Fig. 4(c)–(e)). To analyze this, the FTIR spectrum of poly(cyclohexandimethylene terephthalate) (PCT), which is a polyester consisting only of CHDM and TPA, was obtained, it showing peaks at both 1240 and 1260 cm−1 (Fig. S2†). The peaks at 1240 and 1260 cm−1 come from the cis and trans structure of CHDM the cis/trans ratio for which is 22/78.19 The enhancement of the peak at 1260 cm−1 indicates the alignment of trans configuration of CHDM which can be induced by stretching force. This result agrees well with previous findings.49,50 As a result, for randomly deposited nanofibers, it was hard for C–O stretching to resonate with the polarized IR beam, and consequently the peak at 1240 cm−1 was stronger and the peak at 1260 cm−1 was weaker than for uniaxially well-oriented nanofibers (Fig. 4(c) and (d)).
Conclusions
This study has shown that novel nanofibers of copolyester containing a biobased monomer, isosorbide, with high Tg and good mechanical properties can be produced by electrospinning. We successfully fabricated nanofibers and optimized the diameter of the nanofibers by varying the PEICT concentration and the diameter was adjusted by controlling the viscosity. The fabricated nanofibers showed high Tg compared with the original pellet and the smaller diameter nanofibers showed higher Tg than the larger diameter nanofibers. This could be explained by molecular chain orientation when nanofibers were spun. The enhancement of thermal property of nanofibers is a meaningful result for industrial applications. The tensile property was enhanced as the diameter was increased because molecular chain orientation existed in finer nanofibers.Nanofibers have extremely high specific surface area because of their small diameters which leads to nanofibers having advanced applications in biomedicine, environmental protection, sensors and energy harvesting.51–53 Thus, the enhanced mechanical and thermal properties of polymeric nanofibers containing biobased material could be utilized in industrial applications such as tissue engineering, wound dressings, filtration and health care devices in the future, and more related studies should be conducted.
Notes and references
- A. K. Mohanty, M. Misra and G. Hinrichsen, Macromol. Mater. Eng., 2000, 276–277, 1–24 CrossRef.
- V. Dornburg, I. Lewandowski and M. Patel, J. Ind. Ecol., 2003, 7, 93–116 CrossRef CAS.
- T. Rastogi, C. Leder and K. Kummerer, RSC Adv., 2015, 5, 27–32 RSC.
- Y. Li, G. A. Thouas and Q.-Z. Chen, RSC Adv., 2012, 2, 8229–8242 RSC.
- R. Quintana, A. M. de Ilarduya, A. Alla and S. Muñoz-Guerra, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 2252–2260 CrossRef CAS.
- C. Zhou, Z. Wei, Y. Yu, Y. Wang and Y. Li, RSC Adv., 2015, 5, 68688–68699 RSC.
- C. Besset, J.-P. Pascault, E. Fleury, E. Drockenmuller and J. Bernard, Biomacromolecules, 2010, 11, 2797–2803 CrossRef CAS PubMed.
- H. G. Fletcher and R. M. Goepp, J. Am. Chem. Soc., 1945, 67, 1042–1043 CrossRef CAS.
- R. C. Hockett, H. G. Fletcher, E. L. Sheffield and R. M. Goepp, J. Am. Chem. Soc., 1946, 68, 927–930 CrossRef CAS PubMed.
- R. C. Hockett and E. L. Sheffield, J. Am. Chem. Soc., 1946, 68, 937–939 CrossRef CAS PubMed.
- P. Stoss and R. Hemmer, in Advances in Carbohydrate Chemistry and Biochemistry, ed. H. Derek, Academic Press, 1991, vol. 49, pp. 93–173 Search PubMed.
- F. Fenouillot, A. Rousseau, G. Colomines, R. Saint-Loup and J. P. Pascault, Prog. Polym. Sci., 2010, 35, 578–622 CrossRef CAS.
- S. Chatti, S. M. Weidner, A. Fildier and H. R. Kricheldorf, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 2464–2471 CrossRef CAS.
- R. Vendamme and W. Eevers, Macromolecules, 2013, 46, 3395–3405 CrossRef CAS.
- S. V. S. Frederick and C. Hughes, Environ. Health Perspect., 2005, 113, 926–933 CrossRef.
- Q. Li, W. Zhu, C. Li, G. Guan, D. Zhang, Y. Xiao and L. Zheng, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 1387–1397 CrossRef CAS.
- C.-H. Lee, H. Takagi, H. Okamoto and M. Kato, J. Appl. Polym. Sci., 2013, 127, 530–534 CrossRef CAS.
- W. J. Yoon, S. Y. Hwang, J. M. Koo, Y. J. Lee, S. U. Lee and S. S. Im, Macromolecules, 2013, 46, 7219–7231 CrossRef CAS.
- W. J. Yoon, K. S. Oh, J. M. Koo, J. R. Kim, K. J. Lee and S. S. Im, Macromolecules, 2013, 46, 2930–2940 CrossRef CAS.
- H. R. Kricheldorf and Z. Gomourachvili, Macromol. Chem. Phys., 1997, 198, 3149–3160 CrossRef CAS.
- D. Czaplewski, J. Kameoka and H. G. Craighead, J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct.--Process., Meas., Phenom., 2003, 21, 2994–2997 CrossRef CAS.
- J. Kameoka and H. G. Craighead, Appl. Phys. Lett., 2003, 83, 371–373 CrossRef CAS.
- Z. Khatri, S. Ali, I. Khatri, G. Mayakrishnan, S. H. Kim and I.-S. Kim, Appl. Surf. Sci., 2015, 342, 64–68 CrossRef CAS.
- O. Ohsawa, K.-H. Lee, B.-S. Kim, S. Lee and I.-S. Kim, Polymer, 2010, 51, 2007–2012 CrossRef CAS.
- J. Kameoka, R. Ilik, D. Czaplewski, R. Mathers, G. Coates and H. G. Craighead, J. Photopolym. Sci. Technol., 2004, 17, 421–425 CrossRef CAS.
- H. Ko and J. Kameoka, J. Photopolym. Sci. Technol., 2006, 19, 413–418 CrossRef CAS.
- L. M. Bellan, G. W. Coates and H. G. Craighead, Macromol. Rapid Commun., 2006, 27, 511–515 CrossRef CAS.
- L. D. Santos, C. Laberty-Robert, M. Maréchal, H. Perrot and O. Sel, Langmuir, 2015, 31, 9737–9741 CrossRef CAS PubMed.
- Y. Zhou, D. Yang, X. Chen, Q. Xu, F. Lu and J. Nie, Biomacromolecules, 2008, 9, 349–354 CrossRef CAS PubMed.
- W. Wang, S. Itoh, K. Konno, T. Kikkawa, S. Ichinose, K. Sakai, T. Ohkuma and K. Watabe, J. Biomed. Mater. Res., Part A, 2009, 91, 994–1005 CrossRef PubMed.
- A. Cooper, N. Bhattarai and M. Zhang, Carbohydr. Polym., 2011, 85, 149–156 CrossRef CAS.
- M. Pakravan, M.-C. Heuzey and A. Ajji, Polymer, 2011, 52, 4813–4824 CrossRef CAS.
- M. Ignatova, N. Manolova and I. Rashkov, Macromol. Biosci., 2013, 13, 860–872 CrossRef CAS PubMed.
- X.-J. Huang, D. Ge and Z.-K. Xu, Eur. Polym. J., 2007, 43, 3710–3718 CrossRef CAS.
- L. Eadie and T. K. Ghosh, J. R. Soc., Interface, 2011 DOI:10.1098/rsif.2010.0487.
- H. R. Darrell and C. Iksoo, Nanotechnology, 1996, 7, 216 CrossRef.
- S. Ali, Z. Khatri, K. W. Oh, I.-S. Kim and S. H. Kim, Macromol. Res., 2014, 22, 971–977 CrossRef CAS.
- N. Kimura, H.-K. Kim, B.-S. Kim, K.-H. Lee and I.-S. Kim, Macromol. Mater. Eng., 2010, 295, 1090–1096 CrossRef CAS.
- Q. Yang, Z. Li, Y. Hong, Y. Zhao, S. Qiu, C. Wang and Y. Wei, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 3721–3726 CrossRef CAS.
- Y. J. Ryu, H. Y. Kim, K. H. Lee, H. C. Park and D. R. Lee, Eur. Polym. J., 2003, 39, 1883–1889 CrossRef CAS.
- S. Iwamoto, A. Isogai and T. Iwata, Biomacromolecules, 2011, 12, 831–836 CrossRef CAS PubMed.
- D. Papkov, Y. Zou, M. N. Andalib, A. Goponenko, S. Z. D. Cheng and Y. A. Dzenis, ACS Nano, 2013, 7, 3324–3331 CrossRef CAS PubMed.
- S.-C. Wong, A. Baji and S. Leng, Polymer, 2008, 49, 4713–4722 CrossRef CAS.
- K. Y. Hwang, S.-D. Kim, Y.-W. Kim and W.-R. Yu, Polym. Test., 2010, 29, 375–380 CrossRef CAS.
- D. Braun and M. Bergmann, Journal für Praktische Chemie/Chemiker-Zeitung, 1992, 334, 298–310 CrossRef CAS.
- K. C. Cole, A. Ajji and É. Pellerin, Macromolecules, 2002, 35, 770–784 CrossRef CAS.
- M. C. Tobin, J. Phys. Chem. C, 1957, 61, 1392–1400 CrossRef CAS.
- S. W. Lee, W. Huh, Y. S. Hong and K. M. Lee, Korea Polym. J., 2000, 8, 261–267 Search PubMed.
- M. V. Kakade, S. Givens, K. Gardner, K. H. Lee, D. B. Chase and J. F. Rabolt, J. Am. Chem. Soc., 2007, 129, 2777–2782 CrossRef CAS PubMed.
- K.-H. Lee, K.-W. Kim, A. Pesapane, H.-Y. Kim and J. F. Rabolt, Macromolecules, 2008, 41, 1494–1498 CrossRef CAS.
- C. L. Casper, W. Yang, M. C. Farach-Carson and J. F. Rabolt, Biomacromolecules, 2007, 8, 1116–1123 CrossRef CAS PubMed.
- C. Shin, G. G. Chase and D. H. Reneker, Colloids Surf., A, 2005, 262, 211–215 CrossRef CAS.
- H. J. Kim, Y. S. Kim, M. H. Seo, S. M. Choi and W. B. Kim, Electrochem. Commun., 2009, 11, 446–449 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Relationship between shear viscosity and diameter, SEM images, IR spectrum of PCT are provided. See DOI: 10.1039/c6ra02852g |
| This journal is © The Royal Society of Chemistry 2016 |