A novel & effective visible light-driven TiO2/magnetic porous graphene oxide nanocomposite for the degradation of dye pollutants†
Roya Sedghi* and
Fatemeh Heidari
Department of Polymer, Faculty of Chemistry, Shahid Beheshti University, G.C, 1983969411, Tehran, Iran. E-mail: r_sedghi@sbu.ac.ir; Fax: +98-21-22431661
First published on 6th May 2016
Abstract
Porous graphene oxide (pGO) was applied for the preparation of various nanocomposites, including TiO2 (anatase)/pGO, TiO2 (mix phase)/pGO, TiO2 (anatase)/magnetic pGO and TiO2 (mix)/magnetic pGO, using titanium(IV) chloride as the photocatalyst precursor. Field emission scanning electron microscopy (FESEM), X-ray diffraction (XRD), thermogravimetric analysis (TGA), Fourier transform infrared (FT-IR) spectroscopy, diffuse reflectance UV-vis spectroscopy (DR-UV-vis), and N2 adsorption–desorption using Brunauer–Emmett–Teller (BET) analysis were employed to investigate the morphology, crystal structure, surface groups, and optical properties of the prepared nanocomposites. The photocatalytic performance of the synthesized nanocomposites under visible light was investigated in the degradation of Rhodamine B (RhB) as a model organic pollutant. The TiO2 (mix)/magnetic pGO nanocomposite showed enhanced efficiency under visible light irradiation compared with the other catalysts. 100% degradation using the TiO2 (mix)/magnetic pGO nanocomposite under visible light was achieved in less than 20 min in comparison with TiO2 (mix, anatase) nanoparticles, and TiO2 (anatase)/pGO and TiO2 (mix)/pGO nanocomposites. The degradation efficiency of RhB using the stable nanocomposite remained at 85% after 10 times reuse. The highest photocatalyst performance was achieved with 0.01 g of nanocatalyst and 10 mg L−1 of RhB at pH 9 under visible light irradiation in less than 20 minutes. Moreover, the effect of various scavengers, such as methanol (OH˙ radicals scavenger), disodium ethylenediaminetetraacetate (EDTA, holes scavenger), and p-benzoquinone (BQ, O2˙− radicals scavenger), on RhB degradation was investigated.
1. Introduction
Environmental problems, such as the treatment of pollutants in the air and water, is an important challenge that we are faced with in the world.1 A great issue over a last decade lies in the purification of waste water during industrial, agronomical, and domestic activities.2 Semiconductor photocatalysis has been reported as an impressive method for solving worldwide environmental pollution issues.1 Titanium dioxide as an important semiconductor has been widely studied in the photocatalytic field. It has attracted much attention due to its advantages such as wide compatibility, unique optical characteristics, chemical stability, long durability, nontoxicity, low cost, and electronic properties. These suitable properties also make TiO2 a potential candidate for applications in Li-ion batteries, sensors, solar cells, UV lasers, field-effect transistors. Moreover, its advantages make TiO2 suitable for the decomposition of organic pollutants in air and aqueous media (e.g., organic dyes, toxic micro pollutants and oils) into CO2 and H2O.3–5 TiO2 exists as two main polymorphs, anatase and rutile, in which the rutile phase is known as the “photocatalytically less” active form, while the anatase form is most widely used as a photocatalyst, due to its high surface area and slow charge carrier recombination.6 However, the mixture of anatase and rutile phase exhibits synergetic effects between both phases.6 Nonetheless, the main issue is that the band gap of TiO2 photocatalysts lies in the UV region, which covers less than 5% of the solar spectrum and the photonic efficiency of unmodified monophasic TiO2 is restricted owing to its large band gap (3.2 eV), high charge carrier recombination rate, and low surface area.7,8 Some of the strategies to overcome these limitations and extend its light absorption range include metal (V, Co, Ag, and etc.) and non-metal doping (C, N). Among these dopants carbon has received eminent importance.7,9Within the past decade a new carbonaceous material, “graphene” has attracted much attention, due to its high carrier mobility, unique electronic property, high thermal conductivity, and extremely high theoretical specific surface area (∼2600 m2 g−1).10 Graphene has better conductivity in comparison to other types of carbon materials (e.g., carbon nanotubes, and activated carbon) due to its higher surface area.11 One of the most important derivatives of graphene is graphene oxide (GO).11 GO is a two dimensional sheet with sp2 hybridized orbitals, which is characterized by a layered structure with hydrophilic oxygen functional groups located in the edge and basal plane.3,11 GO can act as an acceptor/transporter of photogenerated electrons for TiO2 nanoparticles (NPs) and reduce the recombination of photogenerated electron–holes, thus resulting in the enhancement of the photocatalytic activity.3,5,12 It is possible to improve the properties of graphene and create new types of graphene-based materials by modifying the structure of graphene (edges or basal planes).13 Recently, porous graphene (pGO), which is a graphene-based material, has stimulated much interest of many researchers because of its potential applications in nanoelectronics, gas sensors, hydrogen storage, water and air purification and many other fields.13 pGO contains holes either with high or random regularity. pGO nanoparticles are the most employed porous materials due to their high surface areas and “space” for the transportation or storage of electrons/ions and gases or liquids with a hydrophobic nature, and their low cost to manufacture.
In the previous decades, Fe3O4 magnetic NPs have attracted great attention due to their excellent adsorption, low toxicity, catalytic properties, unusual structural, and biocompatibility.14 Decorating porous graphene oxide sheets with inorganic materials such as Fe3O4 could result in the formation of an extraordinary 2D nanocomposite structure.15 The combination of Fe3O4 with porous graphene oxide can effectively prevent the agglomeration of individual graphene sheets and it will have a very specific surface area and potential applications in waste water treatment and it can also be a convenient adsorbent for dye pollutants.14 Furthermore, another advantage of this combination is the complete removal of the sorbent by an external magnetic field.16
For the first time, the effectiveness of TiO2 (mix)/magnetic pGO and TiO2 (anatase)/magnetic pGO nanocomposites as photocatalysts for RhB degradation under visible light irradiation is compared. Using these magnetic nanocomposites the photocatalytic efficiency of neat TiO2 nanoparticles was enhanced under visible light irradiation. Some experimental parameters, such as photocatalyst and RhB concentration and pH of the sample, were optimized. The structures of the prepared nanocomposites were characterized using various techniques, including UV-vis spectroscopy, FT-IR, SEM, EDX, XRD, TGA and BET.
2. Experimental
2.1. Reagents and materials
Titanium(IV) chloride (>99%), ammonium sulfate (>99%), ammonium hydroxide (25–30%), FeCl3·6H2O (>99%), FeCl2·4H2O (>99%), 3-aminopropyltrimethoxysilane (APTMS > 97%), oxalic acid dehydrate (>99%), KMnO4, RhB and hydrazine hydrate of analytical grade were obtained from Merck Company (Whitehouse Station, NJ). Furthermore, graphite powder (99.95%) was purchased from Alfa Aesar.2.2. Catalyst characterization
The morphology of the products was characterized using a Cambridge scanning electron microscope (Stereoscan 360). BET analysis was used to determine the specific surface areas of the prepared nanophotocatalysts using a BELSORP-18 Plus (BEL Japan) through N2 adsorption (degassing temperature was at 120 °C for 12 h). XRD measurements were conducted on a XD-3A diffractometer with CuK radiation (λ = 1.5406 Å). Thermogravimetric analysis of the nanocomposites was conducted using a TGA/DTA (BAHR: STA 503) at a heating rate of 10 °C min−1 in air. FT-IR measurements were carried out on a BOMEM MB-series FT-IR spectrometer in the form of dried samples using KBr pellets. A Jencons ultrasonic system was used to disperse the NPs or nanocomposites in an aqueous medium. The concentrations of dyes solutions were determined using a UV-S600 spectrophotometer (Analytikaljena). Samples were irradiated using visible light (180 power LED lamp in a circular manner, 3.2 V, 1 W, (79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Lux)). The intensity was determined using a light meter (MASTECH model MS 6612 digital Lux meter) at λ > 390 nm. UV-vis diffuse reflectance spectra of the samples were obtained on a Shimadzu-2100 UV-vis spectrophotometer in the range of 200–900 nm and BaSO4 was used as a reference material. The pH of the solutions was measured using a Methrohm digital pH meter 827. An inductively coupled plasma optical emission spectrometer (ICP-OES; Varian Vista PRO Radical) was used to determine the content of Ti and Fe in the samples. The magnetic properties of the particles were analyzed using a vibrating sample magnetometer (DC magnetometer 1.5 Tesla) (Imax = 150 A, P ≤ 9 kW) at room temperature.
000 Lux)). The intensity was determined using a light meter (MASTECH model MS 6612 digital Lux meter) at λ > 390 nm. UV-vis diffuse reflectance spectra of the samples were obtained on a Shimadzu-2100 UV-vis spectrophotometer in the range of 200–900 nm and BaSO4 was used as a reference material. The pH of the solutions was measured using a Methrohm digital pH meter 827. An inductively coupled plasma optical emission spectrometer (ICP-OES; Varian Vista PRO Radical) was used to determine the content of Ti and Fe in the samples. The magnetic properties of the particles were analyzed using a vibrating sample magnetometer (DC magnetometer 1.5 Tesla) (Imax = 150 A, P ≤ 9 kW) at room temperature.
2.3. TiO2 NPs synthesis
For the synthesis of the TiO2 NPs, 0.75 mL of TiCl4 was slowly added to a solution of 1.5 M ammonium sulfate. Then, the prepared solution was heated at 75 °C under stirring for 90 min. In the next step, an ammonium hydroxide solution (2.5 M) was added drop by drop under high speed stirring until the pH was 7.0. Then, the white solid precipitate was filtered and repeatedly washed with distilled water and ethanol and dried at 50 °C. Subsequently, the powder was calcined at temperatures of 450 °C and 750 °C for 4 h (for the synthesis of anatase and mix phase TiO2, respectively).172.4. Synthesis of surface modified TiO2
At first, 0.5 g of TiO2 (anatase and mix phase) was added to 40 mL ethanol/water with a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and dispersed in an ultrasonic bath. Then, 1 mL APTMS was added to the solution to form a homogeneous suspension under vigorous stirring, following by refluxing for 12 h at 85 °C. Subsequently, the product was washed with ethanol and then dried sufficiently.
1 ratio and dispersed in an ultrasonic bath. Then, 1 mL APTMS was added to the solution to form a homogeneous suspension under vigorous stirring, following by refluxing for 12 h at 85 °C. Subsequently, the product was washed with ethanol and then dried sufficiently.
2.5. Synthesis of porous GO
GO was prepared via a modified Hummers' method from graphite powder.18 In brief, 0.5 g GO was added to 100 mL deionized water, and dispersed using an ultrasonic bath for 3 h. Then, 1 g KMnO4 was added to the solution, followed by stirring for 15 min. The resulting suspension was heated in household microwave oven for 5 min and then the black product was mixed with 100 mL water and 20 mL hydrazine solution. The reaction mixture was refluxed at 100 °C (24 h) for the reduction of GO. Finally, in the last step, the obtained product was washed several times with oxalic acid and hydrochloric acid (v/v 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), water and ethanol and then dried at 70 °C in a vacuum oven.19
1), water and ethanol and then dried at 70 °C in a vacuum oven.19
2.6. Synthesis of magnetic pGO
To obtain the super paramagnetic pGO nanoscomposite, first 0.5 g pGO was added to 100 mL deionized water and then dispersed using an ultrasonic bath for 1 h. Then, under vigorous stirring 0.25 g FeCl2·4H2O and 0.5 g FeCl3·6H2O with the molar ratio of Fe3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe2+ = 2
Fe2+ = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was added to the solution under an N2 atmosphere.20 Subsequently, 15 mL of ammonia solution (% 25) was added to the suspension at 80 °C. After stirring for 30 min, the precipitates were separated with an external magnetic field and washed several times with water and ethanol, respectively. Then, the product was dried at 35 °C in a vacuum oven.
1 was added to the solution under an N2 atmosphere.20 Subsequently, 15 mL of ammonia solution (% 25) was added to the suspension at 80 °C. After stirring for 30 min, the precipitates were separated with an external magnetic field and washed several times with water and ethanol, respectively. Then, the product was dried at 35 °C in a vacuum oven.
2.7. Synthesis of TiO2/magnetic pGO
First, 0.5 g magnetic pGO and 0.5 g of TiO2-APTMS (in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were added to 100 mL water and dispersed for 30 min in an ultrasonic bath under vigorous stirring. The mixture was refluxed at 100 °C for 24 h. The obtained precipitates were washed with water and ethanol several times. The product was separated with an external magnetic field and dried at 50 °C. The amount of Ti and Fe in the TiO2/magnetic pGO nanocomposite was determined to be 2.23 and 9.57 mmol g−1, respectively, by inductively coupled plasma-optical emission spectroscopy (ICP-OES).
1) were added to 100 mL water and dispersed for 30 min in an ultrasonic bath under vigorous stirring. The mixture was refluxed at 100 °C for 24 h. The obtained precipitates were washed with water and ethanol several times. The product was separated with an external magnetic field and dried at 50 °C. The amount of Ti and Fe in the TiO2/magnetic pGO nanocomposite was determined to be 2.23 and 9.57 mmol g−1, respectively, by inductively coupled plasma-optical emission spectroscopy (ICP-OES).
2.8. Photocatalytic degradation of RhB
The photocatalytic performance of the prepared TiO2 (mix)/magnetic pGO nanocomposite was investigated in degradation of RhB dye under visible light (Fig. 1). A solution of RhB was prepared as an initial solution with a concentration of 10 mg L−1. The 0.01 g photocatalyst was added to 100 mL of the initial RhB solution. The irradiation source was a visible light (LED lamp and λ > 390 nm). The mixture was sonicated in the dark for 5 min and stirred for 30 min to ensure the establishment of adsorption–desorption equilibrium. During the photocatalytic reaction, the mixture was exposed to visible light and stirred by a magnetic stirrer. Subsequently, the analytical samples were taken out from the reactor and immediately centrifuged or separated using an external magnet (for magnetic catalysts) or filtered through a nanofilter to remove the photocatalysts. The transparent solutions were analyzed by recording the variation in absorption in the UV-vis spectrum of RhB using a UV-vis spectrophotometer (λ = 560 nm). | ||
| Fig. 1 Photocatalytic activity test of TiO2/magnetic pGO nanocomposite under visible light irradiation for RhB degradation. | ||
2.9. Detection of reactive oxidative species
The reactive species in the oxidation were detected via in situ trapping experiments during the photodegradation tests. The process of tracing is similar to the photodegradation experimental process. Different scavengers, including methanol (OH˙ radicals scavenger), disodium ethylenediaminetetraacetate (EDTA, holes scavenger), and p-benzoquinone (BQ, O2˙− radicals scavenger), prior to the addition of the photocatalyst in three different photodegradation experiments, were added to the RhB solution and the concentration of the scavengers was 1.0 mM.213. Results and discussion
Scheme 1 shows the synthetic reactions for the TiO2/magnetic pGO nanocomposite as a photocatalyst. The amine functionalized TiO2 NPs react with the functional groups on the pGO sheets and electrons can be transported along the pGO sheets and then the generation of OH˙ from adsorbed O2 and H2O occurs. Accordingly, this effective charge transfer could decrease the charge recombination and increase the photocatalytic activity of TiO2/magnetic pGO nanocomposites.223.1. Characterization of photocatalysts
 | ||
| Fig. 2 SEM images of GO (a), pGO (b), magnetic pGO (c), TiO2/magnetic pGO (d) nanocomposite and (e) EDX element analysis of TiO2/magnetic pGO as the final nanophotocatalyst. | ||
Fig. 2a and b show GO and porous GO, respectively. The graphene oxide layer is clear in these figures. The porous surface of pGO contains holes with random regularity. It can be observed that Fe3O4 NPs and TiO2 NPs are uniformly distributed on the pGO sheets (Fig. 2c and d). In addition, TiO2 and Fe3O4 NPs could sufficiently interact with the functional groups on the pGO sheets, which are well dispersed in an aqueous phase and lead to formation of a uniform mixture of pGO sheets and TiO2 NPs. The micrographs of the nanocomposites Fig. 2c and d show that the average size of the nanoparticles is approximately 50 nm. The nanoparticles have a spherical structure with a smooth surface morphology. The EDX spectra (Fig. 2e) of the final nanocomposite confirmed the presence of expected elements in the obtained product on final stage.
 | ||
| Fig. 3 XRD patterns of TiO2 (mix), TiO2 (anatase), GO, pGO, magnetic pGO and TiO2 (mix)/magnetic pGO nanocomposites. | ||
Diffraction lines of the TiO2 (mix)/magnetic pGO nanocomposites were observed at 2θ = 30.10°, 35.52°, 48.06°, 53.90°, 56.97°, 62.76° and 75°, which can be assigned to the 220, 311, 400, 422, 511, 440 and 533 reflection, respectively, and these show standard Fe3O4 NPs crystals with a spinal structure. The peaks located at 2θ = 25.32°, 37.81° and 55° are due to TiO2 and the absence of the typical peak of pGO is possibly due to the disruption and good exfoliation of pGO in the TiO2 (mix)/magnetic pGO nanocomposites or because of the low intensity of the pGO pattern.27
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration at 1622.10 cm−1, which affirm the presence of oxygen containing functional groups at the edge of the graphene oxide layers. The broad absorption band located in 3370.27 cm−1 is imputed to the remaining –OH groups of pGO. In general, in the comparison of GO nanosheets there is no remarkable difference between these nanosheets and merely some of the functional groups on GO was reduced. In the magnetic pGO spectrum the additional peaks at 1403.24 cm−1 and 1617.96 cm−1 are due to the presence of Fe3O4 in the nanocomposites.10–12 The spectrum of the TiO2/magnetic pGO nanocomposite is similar to the magnetic pGO spectrum. The broad band peak at 3387 cm−1 is assigned to O–H stretching from the hydroxyl groups. Moreover, the peaks at 1569 cm−1, 1483 cm−1, 1135 cm−1 and 685 cm−1 are related to carbonyl C
O stretching vibration at 1622.10 cm−1, which affirm the presence of oxygen containing functional groups at the edge of the graphene oxide layers. The broad absorption band located in 3370.27 cm−1 is imputed to the remaining –OH groups of pGO. In general, in the comparison of GO nanosheets there is no remarkable difference between these nanosheets and merely some of the functional groups on GO was reduced. In the magnetic pGO spectrum the additional peaks at 1403.24 cm−1 and 1617.96 cm−1 are due to the presence of Fe3O4 in the nanocomposites.10–12 The spectrum of the TiO2/magnetic pGO nanocomposite is similar to the magnetic pGO spectrum. The broad band peak at 3387 cm−1 is assigned to O–H stretching from the hydroxyl groups. Moreover, the peaks at 1569 cm−1, 1483 cm−1, 1135 cm−1 and 685 cm−1 are related to carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of pGO, symmetric stretching vibrations of Fe3O4, alkoxy C–O stretching group of pGO, and bending vibration and stretching modes of the TiO2 NPs, respectively.
O stretching vibration of pGO, symmetric stretching vibrations of Fe3O4, alkoxy C–O stretching group of pGO, and bending vibration and stretching modes of the TiO2 NPs, respectively.
The large surface area (395.26 m2 g−1) is due to the porosity of pGO and its small pore size can make more active sites for nanocomposites. The large SBET of the TiO2/magnetic pGO nanocomposite indicates a good catalytic performance.
3.2. Photocatalytic activity
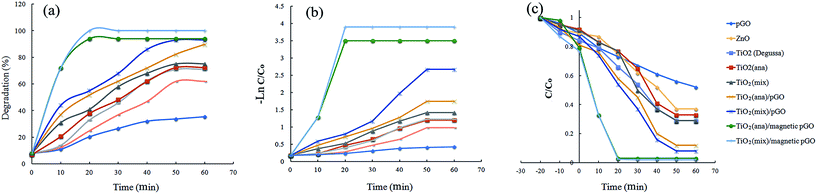
Fig. 8a shows the photocatalytic activity of TiO2 (anatase), TiO2 (mix) nanoparticles and TiO2 (anatase)/pGO, TiO2 (mix)/pGO, TiO2 (anatase)/magnetic pGO and TiO2 (mix)/magnetic pGO nanocomposites under visible light irradiation. In the presence of each pure TiO2 (anatase and mix), there is a low degradation efficiency for RhB due to their wide band gap (3.2 eV for anatase and >3.2 eV for mix phase).24 However, the efficiency of the mix phase is a little better than the monophasic TiO2, because the presence of the rutile phase (in addition to the anatase phase) better results are obtained because the rutile phase may be activated by visible light.6,30 Therefore, the degradation efficiency of the RhB solution (in 60 min) over pure TiO2 NPs in anatase and mix phase is 72% and 75%, respectively. However, the efficiency of degradation was significantly recovered under the same conditions by the loaded pGO and magnetic pGO nanocomposites. The photocatalytic efficiencies of the TiO2 (mix)/pGO, TiO2 (anatase)/pGO, TiO2 (anatase)/magnetic pGO and TiO2 (mix)/magnetic pGO nanocomposites were increased to 93% and 89% in 60 min and 95% and 99% in 20 min, respectively. The degradation of RhB by TiO2 (Degussa), ZnO and porous graphene oxide compared to TiO2/magnetic porous graphene oxide confirmed the highest performance of the TiO2/magnetic porous graphene oxide. Furthermore, the results of our investigation were compared with previous reports of RhB degradation from reaction times and performance viewpoints (Table 1).The Fig. 8c shows the relationship between C/C0 and reaction time in dark conditions and visible light irradiation for RhB. As observed in the graphs, there is no significant adsorption in the dark condition. However, it could be observed, for the TiO2 (anatase)/magnetic pGO and TiO2 (mix)/magnetic pGO nanocomposites, the amount of adsorption increased and this due to the effective presence of pGO and magnetic pGO.
The characterization results show that the optical response of TiO2 shifted to the visible region from the UV region with the introduction of Fe3O4 and pGO nanoparticles. The reaction rate of different catalysts can be compared using the pseudo-first-order reaction equation:
| −dC/dt = kC |
The concentration of RhB at time “t” is “C” and the apparent rate reaction constant is k. Therefore, the equation can be integrated to give the following form:8
| −ln(C/C0) = kt |
Therefore, Fig. 8b shows the relationships between in −ln(C/C0) and irradiation time for RhB degradation. The linear relationships (≥99%) in the photocatalyst curves demonstrate that the degradation of RhB is well matched with first order kinetics. The TiO2 (mix)/magnetic pGO nanocomposite displays the highest rate constant (k = 0.2), in all the nanocomposites, which was nearly 10 times larger than the pure anatase TiO2 and even larger than the TiO2 (anatase)/magnetic pGO nanocatalyst (k = 0.17).
3.3. Photocatalyst stability
An important factor for practical applications is the stability of photocatalysts. In the photodegradation of RhB over the TiO2 (mix)/magnetic pGO photocatalyst, cycling runs were conducted, as shown in Fig. 9a. After 10 runs, the photocatalytic activity of the nanocomposite slightly decreased. The photodegradation efficiency of RhB is about 85%, which indicates that the photocatalyst is stable. The XRD pattern and IR analysis of nanocatalyst after 10 times usage shows the same peaks and only small decreases in the peak intensity are shown (Fig. 9b and c).3.4. Photodegradation mechanism of TiO2/magnetic pGO
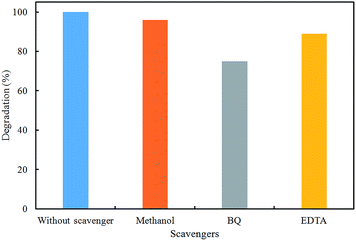
To understand the active species in the degradation of RhB, the effect of several radical scavengers, such as methanol (OH˙ radicals scavenger), disodium methylenediaminetetraacetate (EDTA, holes scavenger) and p-benzoquinone (BQ, O2˙− radicals scavenger), was tested to determine the reaction mechanism.21 Fig. 10 shows the photocatalytic degradation of RhB using the TiO2/magnetic pGO nanocomposite in the presence of different scavengers. As shown in Fig. 10 with the addition of O2˙− as the radical scavenger (p-benzoquinone), the RhB degradation was depressed evidently, which suggests that the O2˙− radical was main active species in the mechanism. Therefore, the rate of electron–hole pair recombination was mainly reduced in the nanocomposites and thus the photocatalytic activity was improved.The possible mechanism of TiO2/magnetic pGO under visible light irradiation is proposed in Fig. 11. During the photocatalytic oxidation process, when visible light irradiated on the TiO2 NPs surface, holes and electrons were generated. Furthermore, the presence of Fe3O4 NPs caused the conduction band of TiO2 go to higher level, thus the gap energy decreased. In the pure TiO2 NPs, the recombination of hole and electrons occurred quickly and therefore the activity of the photocatalyst decreased.12,31
 | ||
| Fig. 11 Mechanism of the TiO2/magnetic pGO nanocomposite to enhance photocatalytic activity under visible light irradiation. | ||
In the TiO2 (mix)/magnetic pGO nanocomposite, the electrons can transport along the pGO sheets and this is due to the fact that the potential of graphene oxide is below the conduction band of TiO2.12 The porous graphene oxide sheets can promote the effective charge separation of electron–hole pairs. The trapped electrons on the pGO sheets react with adsorbed O2 to generate reactive oxygen species (O2˙−), which react with water to produce hydroxyl radicals. Subsequently, the hydroxyl radicals degrade the dye (RhB). At this time, the holes on the valence band of TiO2 combine with H2O to generate active OH˙. In addition, reactive holes on TiO2 react with adsorbed water or hydroxyl groups to produce surface hydroxyl radicals, which are the reactive species for degrading RhB dye and the holes can oxidize RhB molecules directly.32–37
The main reactions are shown below:
| TiO2 + hν → TiO2 (h+ + e−) |
| TiO2 (h+) + H2O → H+ + OH− |
| TiO2 (e−) + GO → GO (e−) |
| GO (e−) + O2adsorb → GO + O2−˙ |
| O2−˙ + H2O → OH˙ |
| OH˙ + dye → degradation product |
These very active species (OH˙, O2˙−, e−, and h+) efficiently degrade RhB and turn RhB into the less harmful H2O and CO2. In the photocatalytic reaction process, charge recombination is suppressed in the TiO2 NPs, resulting in the enhanced photocatalytic activity of the TiO2/magnetic pGO nanocomposite.
3.5. Chemical oxygen demand (COD) analysis
COD analysis was carried out to confirm the mineralization of RhB dye and the COD value was analyzed. After 20 min irradiation time, the percentage of COD was about 97.4%. It can be observed that the degradation was slower than the decolorization of the dye, which may be due to the breaking the chromophoric group of the dye molecule by the photogenerated oxidants. These results indicate that mineralization of the dye required a little longer irradiation time. Therefore, the activity of the catalyst is convenient and organic pollutants are degraded in an aqueous medium.383.6. Optimization of reaction conditions
4. Conclusion
In summary, various nanocomposites of TiO2 (mix)/magnetic pGO, TiO2 (anatase)/magnetic pGO, TiO2 (mix)/pGO and TiO2 (anatase)/pGO were successfully prepared and their properties were characterized. After quantitative analysis, TiO2 (mix)/magnetic pGO exhibited the best photocatalytic performance for the degradation of RhB, due to its high surface area and the pore volume of pGO in addition to the favorable TiO2 (mix) nanoparticles in the presence of visible irradiation. The presence of pGO as an electron acceptor results an excellent optical property. Therefore, the lifetime of photo-generated electron–hole pairs was significantly increased. The aim is the effective oxidation of RhB under the visible light irradiation. The degradation efficiency of RhB after reusing of the nanocatalyst 10 times remained over 85%. Furthermore, the resulting photocatalyst is able to be separated with an external magnet easily and can be reused several times (because of the presence of Fe3O4 nanoparticles in the nanocomposite). All these advantages demonstrate that this nanocomposite can be a convenient photocatalyst in the purification of environmental organic pollutants.References
- S. W. Lee and W. M. Sigmund, Chem. Commun., 2003, 780–781 RSC.
- Y. Ni, W. Wang, W. Huang, C. Lu and Z. Xu, J. Colloid Interface Sci., 2014, 428, 162–169 CrossRef CAS PubMed.
- P. T. Nguyen, C. Salim, W. Kurniawan and H. Hinode, Catal. Today, 2014, 230, 166–173 CrossRef CAS.
- K. R. Reddy, V. G. Gomes and M. Hassan, Mater. Res. Express, 2014, 1, 015012 CrossRef.
- Y. Gao, M. Hu and B. Mi, J. Membr. Sci., 2014, 455, 349–356 CrossRef CAS.
- M. Boehme and W. Ensinger, Nano-Micro Lett., 2011, 3, 236–241 CrossRef CAS.
- S. Yu, H. J. Yun, Y. H. Kim and J. Yi, Appl. Catal., B, 2014, 144, 893–899 CrossRef CAS.
- R. Kavitha and L. G. Devi, J. Environ. Chem. Eng., 2014, 2, 857–867 CrossRef CAS.
- H. G. Lee, G. Sai-Anand, S. Komathi, A. I. Gopalan, S. W. Kang and K. P. Lee, J. Hazard. Mater., 2015, 283, 400–409 CrossRef CAS PubMed.
- H. Sun, S. Liu, S. Liu and S. Wang, Appl. Catal., B, 2014, 146, 162–168 CrossRef CAS.
- Y. Cong, M. Long, Z. Cui, X. Li, Z. Dong, G. Yuan and J. Zhang, Appl. Surf. Sci., 2013, 282, 400–407 CrossRef CAS.
- P. Muthirulan, C. N. Devi and M. M. Sundaram, Ceram. Int., 2014, 40, 5945–5957 CrossRef CAS.
- P. Russo, A. Hu and G. Compagnini, Nano-Micro Lett., 2013, 5, 260–273 CrossRef.
- W. Lü, Y. Wu, J. Chen and Y. Yang, CrystEngComm, 2014, 16, 609–615 RSC.
- X. Yan, Y. Li, F. Du, K. Zhu, Y. Zhang, A. Su, G. Chen and Y. Wei, Nanoscale, 2014, 6, 4108–4116 RSC.
- G. Z. Kyzas, N. A. Travlou, O. Kalogirou and E. A. Deliyanni, Materials, 2013, 6, 1360–1376 CrossRef CAS.
- Z. Wang, C. Chen, F. Wu, B. Zou, M. Zhao, J. Wang and C. Feng, J. Hazard. Mater., 2009, 164, 615–620 CrossRef CAS PubMed.
- M. R. Nabid, M. Golbabaee, A. B. Moghaddam, R. Dinarvand and R. Sedghi, Int. J. Electrochem. Sci., 2008, 3, 1117–1126 CAS.
- D. Fan, Y. Liu, J. He, Y. Zhou and Y. Yang, J. Mater. Chem., 2012, 22, 1396–1402 RSC.
- Z. Yuanbi, Q. Zumin and J. Huang, Chin. J. Chem. Eng., 2008, 16, 451–455 CrossRef.
- C. Cui, Y. Wang, D. Liang, W. Cui, H. Hu, B. Lu, S. Xu, X. Li, C. Wang and Y. Yang, Appl. Catal., B, 2014, 158, 150–160 CrossRef.
- B. H. Nguyen, V. H. Nguyen and D. L. Vu, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2015, 6, 033001 CrossRef.
- R. J.-N. Z. Xiu-Jian and L. B.-S. H. Xin, Chin. J. Inorg. Chem., 2006, 3, 029 Search PubMed.
- A. Ramchiary and S. Samdarshi, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2014, 305, 33–39 CAS.
- P. Gao, K. Ng and D. D. Sun, J. Hazard. Mater., 2013, 262, 826–835 CrossRef CAS PubMed.
- Z. Fan, Q. Zhao, T. Li, J. Yan, Y. Ren, J. Feng and T. Wei, Carbon, 2012, 50, 1699–1703 CrossRef CAS.
- J. Jing, Y. Zhang, W. Li and W. Y. William, J. Catal., 2014, 316, 174–181 CrossRef CAS.
- Y. Shi, K. Zhou, B. Wang, S. Jiang, X. Qian, Z. Gui, R. K. Yuen and Y. Hu, J. Mater. Chem. A., 2014, 2, 535–544 CAS.
- Z. Wang, L. Yin, Z. Chen, G. Zhou and H. Shi, J. Nanomater., 2014, 2014, 1–6 Search PubMed.
- S. Bakardjieva, J. Šubrt, V. Štengl, M. J. Dianez and M. J. Sayagues, Appl. Catal., B, 2005, 58, 193–202 CrossRef CAS.
- S. Morales-Torres, L. M. Pastrana-Martínez, J. L. Figueiredo, J. L. Faria and A. M. Silva, Environ. Sci. Pollut. Res., 2012, 19, 3676–3687 CrossRef CAS PubMed.
- U. I. Gaya and A. H. Abdullah, Progress and problems, J. Photochem. Photobiol., C, 2008, 9, 1–12 CrossRef CAS.
- K. Maeda, J. Photochem. Photobiol. C, 2011, 12, 237–268 CrossRef CAS.
- T. Ochiai and A. Fujishima, J. Photochem. Photobiol. C, 2012, 13, 247–262 CrossRef CAS.
- H. Park, Y. Park, W. Kim and W. Choi, J. Photochem. Photobiol. C, 2013, 15, 1–20 CrossRef CAS.
- B. T. Zhang, X. Zheng, H. F. Li and J. M. Lin, Anal. Chim. Acta, 2013, 784, 1–17 CrossRef CAS PubMed.
- X. An and C. Y. Jimmy, RSC Adv., 2011, 1, 1426–1434 RSC.
- D. Maruthamani, D. Divakar and M. Kumaravel, J. Ind. Eng. Chem., 2015, 30, 33–43 CrossRef CAS.
- S. Yang, Y. Huang, Y. Wang, Y. Yang, M. Xu and G. Wang, Int. J. Photoenergy, 2012, 2012, 1–6 Search PubMed.
- L. You-ji and C. Wei, Catal. Sci. Technol., 2011, 1, 802–809 Search PubMed.
- N. Hariprasad, S. Anju and E. Yesodharan, Res. J. Mater. Sci., 2013, 2320, 6055 Search PubMed.
- S. Lei, L. Lin, M. Jun, M. Yanan, Z. Shifa, W. Fangxiao and S. Jianmin, Ceram. Int., 2014, 40, 3495–3502 CrossRef.
- S. Penghui, T. Jiayu, Z. Zhiwei, S. Wenxin, G. Shanshan and C. Fuyi, Appl. Surf. Sci., 2015, 324, 35–43 CrossRef.
- Z. Zhijun, X. Changsheng, Z. Shasha, Y. xueli, Z. Tao and L. Jie, J. Alloys Compd., 2013, 581, 385–391 CrossRef.
- M. Kalantari, M. Kazemeini and A. Arpanaei, Mater. Res. Bull., 2013, 48, 2023–2028 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra02827f |
| This journal is © The Royal Society of Chemistry 2016 |