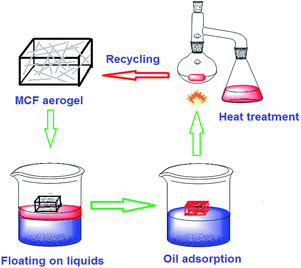
Eco-friendly fabrication of sponge-like magnetically carbonaceous fiber aerogel for high-efficiency oil–water separation†
Rui-Lin Liua,
Xing-Qiang Lib,
Hui-Qin Liub,
Zhi-Min Luoa,
Jin Mac,
Zhi-Qi Zhangc and
Qiang Fu*a
aSchool of Pharmacy, Xi'an Jiaotong University, Xi'an 710061, PR China. E-mail: fuqiang@mail.xjtu.edu.cn; Fax: +86-29-82655382
bSchool of Stomatology, Xi'an Jiaotong University, Xi'an 710061, PR China
cSchool of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an 710062, China
First published on 18th March 2016
Abstract
To handle oil spillage and chemical leakage accidents, the development of novel sorbent materials is of global significance for environment and water source protection. In this work, a magnetically carbonaceous fiber (MCF) aerogel was for the first time fabricated by a facile approach from natural cotton as a precursor, and this material can be used as a potential adsorbent without any further chemical modification for oil–water separation under demanding conditions. Owing to its unique and superior properties, such as twisted fiber structure, light weight, high porosity, desirable hydrophobicity, excellent separation efficiency, and strong thermal/mechanical stability, the MCF aerogel exhibits a high adsorption capacity for organic solvents and oils (22–87 times its own weight) and good recyclability. Coupled with the simple, low-cost, and environment-friendly synthesis process, the MCF aerogel will be a promising candidate for removing organic pollutants in environmental pollution cleanup. Hopefully, the MCF aerogel and the corresponding fabrication approach will be further applied to extensive applications including energy storage, fabrication of multifunctional composite materials, and so on.
1. Introduction
With growing global demand for offshore oil production, oil spills become a risk during exploration and transportation, and may cause serious environmental problems.1–4 In the current situation, the development of novel methods for separation and removal of oil or organic pollutants from water are extremely urgent. Generally, mechanical, biological and chemical clean up as commonly used strategies are used for minimising and mitigating the effects of oil spills.5–7 Mechanical clean up involves the use of equipment and machineries, such as booms, barriers, skimmers and sorbent materials to facilitate the capturing and storage of spilled oil. Biological treatment is used for the biodegradation of oil components contains using the microorganisms.8 However, this method showed poor removal of oil and conflicting results in the literature.9 The chemical method involves combustion of the floating oils, and the use of dispersing agents and solidifiers. Although the burnings are often considered as the last resort, it is not environmentally benign because of the enormous production of soot and greenhouses.9 In addition, the performance of dispersants is still far from being precisely quantified. The spatial variations and heterogeneities of the dispersants concentration results are the basis challenge.10 Amongst all the methods used for oil remediation, the mechanical extraction of oil by sorbents is considered as one of the most efficient and cost effective methods, which can facilitate the sorption process given their large sorption capacity, high uptake rate, and potential recyclability.1,4,7,11To date, a wide range of materials including natural adsorbents, synthetic microporous polymers and carbon-based materials have been used for oil spill removal.7 Due to the excellent adsorption characteristics and fast adsorption kinetics, carbon-based materials12–15 and microporous polymers16–21 have significant advantages over natural adsorbents. However, the small size of the unassembled carbon-based powder materials is difficult to recycle after use, which can cause serious secondary pollution and raise the use-cost.22 The polymers degrade very slowly compared with natural materials, and their environmental and ecological impact remains unclear.7
Over the past years, carbon-based three-dimensional (3D) networks with outstanding properties have been extensively investigated and shown much potential in catalyst supports, gas sensors, energy storage and conversion devices.23 In particular, their low density, high porosity and hydrophobic properties make them attractive candidates for the removal of pollutants and the separation of oil–water. Till now, four types of carbon-based 3D frameworks have been proposed for oil–water separation such as carbonized organic aerogels,4,24 carbon nanotube (CNT) sponges,25,26 spongy graphene,3,27 and carbon nanofiber aerogels.7,28 However, the organic aerogels are fragile and dense in weight, and thus are not effective in oil absorption.4,24 In addition, the fabrication of CNT sponge needs expensive precursors and complex equipment that obstruct their huge production for practical applications.25,26 Graphene-based aerogels could be used for sorption of various oils and organic solvents, but the use of vast chemicals and production of acidic waste during the preparation of GO seriously restrict their industrialization.4 A template-directed hydrothermal carbonization process was used for fabrication of carbonaceous nanofiber aerogels, but the complicated fabrication processes to be still inevitable, the costly templates must be removed and the samples should be carefully dried via freeze drying.7,29,30 Although these novel carbon-based 3D materials can be used for oil–water separation, the tedious work for purifying oil absorbents restrains their application. In contrast, magnetic materials can be placed on the polluted water zone and subsequently separated by an external magnetic field, which arouses much interest for fabricating a magnetic oil absorbent.31–33 Thus, the combination of relatively cheap raw material and a simply scalable approach seems to be a potential alternative for addressing the bottleneck. All these push us to explore a facile, economical and environmentally friendly strategy to massive fabrication of multifunctional carbon-based aerogel for on-demand oil–water separation.
Herein, we for the first time reported a facile approach to fabricate magnetically carbonaceous fiber (MCF) aerogel using natural cotton and ferric chloride hexahydrate as a precursor and magnetic source, respectively. Raw cotton was used as the carbon source, because of its abundant, sustainable and environmentally friendly and contains 90–95% cellulose;4,34 the most important reason is that its can be easily adsorbed heavy metals,4 making it a promising raw material for fabrication of magnetic carbonaceous fiber aerogel. Results showed that the as-made MCF aerogel can absorb a wide range of organic solvents and oils with a maximum sorption capacity up to 87 times the weight of the pristine MCF aerogel. Moreover, the MCF aerogel also exhibits the excellent recyclability, and maintains a high sorption capacity even after five cycles through distillation, burning or squeezing. It is believe that such natural cotton-derived novel MCF aerogel with good performance will have great potential for industrial applications and environmental protection.
2. Experimental section
2.1 Materials and chemicals
Raw cotton and colza oil were obtained from a local supplier in Xi'an, China. FeCl3·6H2O (97.0%), n-hexane, Sudan III, chloroform, dodecane, DMF, acetone, toluene, THF, ethanol and other chemicals were purchased from the Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All chemicals were of analytical grade. Pump oil, diesel oil and gasoline were supplied by Yanchang Petroleum Group (Shaanxi, China).2.2 Fabrication of the magnetically carbonaceous fibers aerogel
Briefly, cylindrical shaped pieces of raw cotton were rinsed several times by deionized water, and then dried in vacuum at 60 °C for 12 h. After that, the dried cotton (20 g) was completely submerged in a 400 mL alcohol solution containing 0.2 M FeCl3·6H2O for 3 days at 60 °C. In this process, the –OH and –CHO groups on the cotton surfaces easily coordinated with adsorbed Fe ions, as the solvent gradually evaporated, and yellowish-brown Fe-based cotton fibers were obtained after drying at 80 °C. The Fe-based cotton fibers were carbonized at 800 °C at a heating rate of 5 °C min−1 for 3 h under nitrogen. Finally, the furnace was cooled down to room temperature naturally to obtain the original MCF aerogel (Fig. S1†). The resultant MCF aerogel was rinsed with deionized water and anhydrous ethanol to remove uncoordinated Fe ions and organic impurities, and dried at 90 °C for 24 h, to give low-density MCF aerogel. For comparison of the surface physical parameter, another carbon aerogel was synthesized by direct carbonization of cotton (DCC) under the same conditions.2.3 Oil/organic solvents–water separation experiments
Oils including pump oil, colza oil, diesel oil and gasoline were dyed with Sudan III and then poured onto a water surface in a container. In a typical sorption test, the MCF aerogel was placed in contact with an organic liquid until the aerogel was completely filled with the organic liquid, and then taken out by a magnet bar for evaluating the adsorption property. In order to avoid evaporation of the absorbed organic liquid, especially for those with low boiling points, the weight measurement should be done quickly. The oil-absorption capacity K of the MCF aerogel was calculated by the ratio between the maximum absorbed oil quantity moil and the MCF aerogel' mass mMCF (i.e., K = moil/mMCF). Then the volume-based absorption capacity was given by the equation Voil/VMCF = (moilρMCF)/(mMCFρoil),31 where ρMCF and ρoil are the density of the MCF aerogel and the oils, respectively.2.4 Characterization
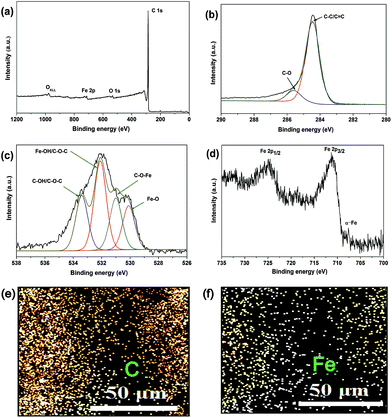
Morphologies of the MCF and DCC aerogels were observed by using a FEI Quanta 200 scanning electron microscopy (SEM). The microstructure of the MCF aerogel was characterized by a JEOL JEM-2100 transmission electron microscopy (TEM). Samples of the MCF aerogel powder were dispersed in ethanol with ultrasonic bath and then a drop of the suspension were placed on a copper grid covered by a holey carbon film. A Rigaku D/Max-3C X-ray diffractometer with Cu Kα1 radiation (λ = 1.54 Å) and an Inel CPS 120 hemispherical detector was used for obtaining the X-ray diffraction (XRD) patterns. Brunauer–Emmett–Teller (BET) surface area of the MCF and DCC aerogels were measured by an ASAP 2020 (Micromeritics). Pore size distributions (PSDs) were determined from the adsorption branches of the isotherms using nonlocal density functional theory (NLDFT) model. The PSD for mesoporous was assessed from the desorption branches of the isotherms using Barrett–Joyner–Halenda (BJH) model. Thermal gravimetric analysis (TGA) was carried out using an America TA Instruments Q50 thermoanalysis instrument in an air and nitrogen flow. Fourier transform infrared spectroscopy (FTIR) spectra were measured using a Bruker Tensor 27 spectrometer. Elemental compositions of the MCF aerogel was determined from energy dispersive X-ray (EDX) analysis attached with SEM. An Axis Ultra X-ray photoelectron spectrometer (XPS; Kratos Analytical Ltd.) was used for obtaining the X-ray photoelectron spectra (XPS). A MPMS-XL-7 superconducting quantum interference device magnetometer (SQUID, Quantum Design) was used for measuring the magnetic property. The contact angle of the MCF aerogel was measured by an OCA20 (Data physics Instruments).3. Results and discussion
3.1 Characterization of MCF aerogel
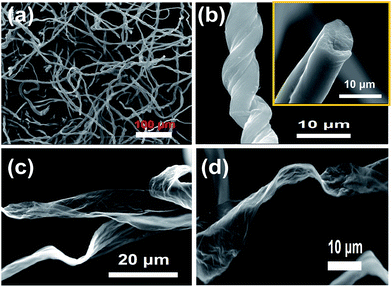
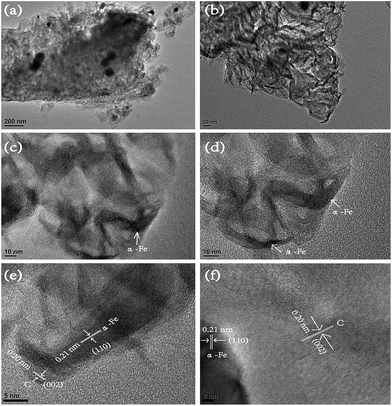
A few pieces of purified raw cotton with cylindrical shape was processed with 0.2 M FeCl3 alcohol solution and pyrolyzed at 800 °C for 3 h under nitrogen atmosphere to generate black and lightweight MCF aerogel. After treatment and pyrolysis, the height of the cylindrical cotton decreased from 6.5 cm to 3.3 cm (Fig. S2a and b†) and the diameter decreased from 2.8 cm to 1.5 cm in comparison to the raw cotton. The SEM image shows that the raw cottons are very long and most of them are up to a few centimeters or longer (Fig. S2c†), and the raw cotton fibers with a diameter of 20–30 μm in raw cotton are slightly twisted with a pitch length of 50–200 μm (Fig. S2d†), which are very close to the previous results.4 In contrast, the DCC aerogel shows a twisted feature and a smaller diameter of 10–15 μm (Fig. S3a†). The MCF aerogel shows a 3D structure and microbelt texture with a smaller diameter of 8–12 μm and a further twisted morphology with a pitch length in the range of 10–20 μm (Fig. 1a and b). In addition, the MCF aerogel contains part of silk-like sheet structures with wrinkled crevice (Fig. 1c and d).The TEM images show that the DCC aerogel has a helix-like feature (Fig. S3b†). In comparison, the MCF aerogel has a fibrous-network structure (Fig. 2a and b). The difference in morphology may be ascribed to the absence and presence of FeCl3, respectively. In general, FeCl3 easily dehydrates carbohydrate polymers at high temperatures and can change the decomposition pathway of lignocellulosic biomass during fast pyrolysis, thus improving the formation of open pores and fibrous-like structure in the carbon matrix. Detailed information on the structure was further obtained using HRTEM. The Fe particles were surrounded by carbon layers (Fig. 2c and d), and the lattice fringe with a distance of 0.21 nm, indicating the (110) plane of the α-Fe crystal. The outer lattice fringe with a calculated d-spacing of 0.20 nm corresponded to the (002) plane of graphite (Fig. 2e and f).35
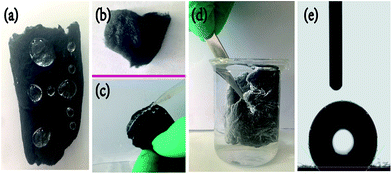
Raw cotton can effectively absorb water (Fig. S4†) because of its hydrophilicity, but the MCF aerogel can support some spherical water droplets on its surface due to its hydrophobic (Fig. 3a). A colza oil droplet dripped on the surface of the MCF aerogel, it can spread very quickly (Fig. 3b). In contrast to hydrophobic wettability to water, the MCF aerogel shows excellent oleophilic property. Interestingly, a jet of water easily bounces on the hydrophobic MCF aerogel, which can be ascribed to the existence of an air cushion between the water and fabric (Fig. 3c).36 Moreover, a mirror-like phenomenon can be observed when the hydrophobic MCF aerogel is pressed into water, further proving the air cushion between the entrapped air in the 3D aerogel and the surrounding water (Fig. 3d).4,30 Meanwhile, when the MCF aerogel was immersed into the water and took out from the water, no water droplets are adhered on the surface in the rising process because they easily roll off, just like a water droplet rolls off a lotus leaf. In addition, the DCC aerogel has similar hydrophobic and oleophilic properties (data not shown), which is consistent with the previous results.4
The FTIR analysis was further used for validating the different wettability between the cotton and MCF aerogel. Results showed that the raw cotton contains several peaks of hydrophilic functional groups, such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O, and –OH (Fig. S5†). In contrast, the resultant MCF aerogel showed very weak signals in C
O, C–O, and –OH (Fig. S5†). In contrast, the resultant MCF aerogel showed very weak signals in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O functional groups, which resulted in its hydrophobicity. A water contact angle (WCA) measurement was performed to thoroughly investigate the surface wettability of the MCF aerogel; it exhibited surface hydrophobicity with a WCA of 132.8° (Fig. 3e), which further confirms the above-mentioned results. Thus, the MCF aerogel exhibits high hydrophobicity and superoleophilicity at ambient temperature without the modification of low-surface-energy chemicals, which is crucial for selective uptake of spilled oils.
O and C–O functional groups, which resulted in its hydrophobicity. A water contact angle (WCA) measurement was performed to thoroughly investigate the surface wettability of the MCF aerogel; it exhibited surface hydrophobicity with a WCA of 132.8° (Fig. 3e), which further confirms the above-mentioned results. Thus, the MCF aerogel exhibits high hydrophobicity and superoleophilicity at ambient temperature without the modification of low-surface-energy chemicals, which is crucial for selective uptake of spilled oils.
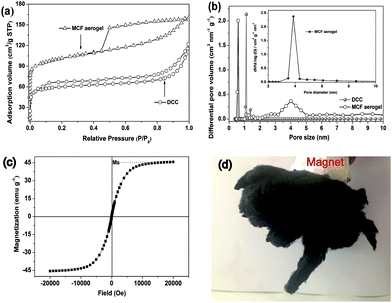
The density of the MCF aerogel was measured to be ca. 36 mg cm−3 by Archimedes' principle (Fig. S6†), which is comparable to that of hydrophobic nanocellulose aerogels (20–30 mg cm−3)37 and lower than the reported hybrid inorganic–organic magnetic foams (50–70 mg cm−3),38 3D graphene/nanoparticle aerogel (42 mg cm−3),39 and monolithic Ni–SiO2, Fe2O3/Fe3O4–SiO2, and NiFe2O4–SiO2 nanocomposites (50–60 mg cm−3),40 but is dramatically lower than those of other ultralight magnetic aerogel and foams including Nd2Fe14B–SiO2 aerogels (140–200 mg cm−3),41 macroporous P(St-DVB)-magnetite nanocomposite (109–233 mg cm−3),42 magnetic shape memory polymer foam (164–218 mg cm−3),43 magnetic microcellular SiOC foam (260–450 mg cm−3),44 iron particle reinforced foams (60–100 mg cm−3)45 and cellulose–CoFe2O4 aerogels (218–390 mg cm−3).46 As a result, a piece of the MCF aerogel could effortlessly stand on a flower without deforming it (Fig. S7a†). Moreover, the MCF aerogel also exhibited good mechanical property, which is beneficial to practical applications. As shown in Fig. S7b–e,† a weight with weight of 100 g was placed on top of the raw cotton and the MCF aerogel accordingly. It can be obviously seen that the raw cotton deformed by ∼80% in height, while the MCF aerogel only deformed by ∼36%. Moreover, the MCF aerogel showed excellent fire resistance (Fig. S8†) when it was exposed to flame.
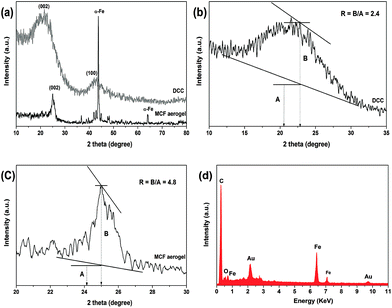
The crystal structure of the MCF and DCC aerogels were determined by using XRD. As shown in Fig. 4, the XRD pattern showed that has strong and broad diffraction peaks of 2θ ≈ 25° and 44.8° for DCC aerogel, which can be attributed to the (002) and (100) diffraction peaks of graphitized carbon,47 respectively. The XRD peaks at 2θ = 44.3° and 64.7° for MCF aerogel indicate the (110) and (200) diffraction peaks of body-centered cubic α-Fe (JCPDS, no. 87-0722).35a This implies that metallic α-Fe nanoparticles formed during the carbonization process by the carbothermal reduction of FeCl3 under an inert atmosphere. The rest of the diffraction peaks are characteristic of the crystalline planes of Fe3C species (JCPDS, no. 89-2867).35b
Dahn et al.48 used the empirical parameter (R) for measure of the number of carbon sheets arranged as single layers, which is defined as the ratio of height of the (002) Bragg peak to the background. A larger R value indicates a higher degree of the graphitization. The R values of MCF (R = 4.8) is obviously larger than that of DCC aerogel (R = 2.4) (Fig. 4b and c), because a relatively higher activation temperature during the Fe catalyst process may lead to some edge orientation and reduce the concentration of nonparallel single layers. Thus, the Fe catalyst process could result in a higher degree of graphitization of the structure, which is very similar with previous result.49 Therefore, the as-obtained material with graphitic character is conduced to further wider applications. EDX analysis shows that the MCF aerogel surface is composed mainly of C and Fe elements (Fig. 4d); which further confirms the XRD results.
TG analysis was used to determine the chemical composition and thermostability of the MCF aerogel, at a heating rate of 10 °C min−1 in an air and nitrogen atmosphere, respectively (Fig. S9†). The first weight loss of MCF aerogel was because of the loss of surface water molecules by heating the material from room temperature to 100 °C. The continuous weight loss of material was measured to be 40%, which occurred at temperatures ranging from 600–1000 °C, likely due to further carbonization of the remaining organics. The content of Fe in the MCF aerogel was evaluated by TGA analysis (Fig. S9†). Ultimately, the carbon became CO2 gas and the α-Fe nanoparticles were oxidized into Fe2O3 after combustion in air. The results proved that the content of Fe2O3 was ∼32.6 wt%. Thus, the α-Fe content was calculated to be ∼22.8 wt%. Notably, the MCF aerogel exhibited higher thermostability up to 500 °C and 600 °C in an air and nitrogen atmosphere accordingly.
As shown in Fig. 5a, XPS of MCF aerogel clearly reveals three peaks, from C1s (284.5 eV), O1s (532.1 eV), and Fe2p (711 eV).47,50 The C1s spectrum of MCF aerogel includes two peaks, with binding energies that can be differentiated via deconvolution (Fig. 5b). These peaks can be assigned to the carbon atoms in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C–C (284.5 eV) and sp3 C–C/C–O (285.5 eV).50,51 Fig. 5c shows the O1s spectrum of MCF aerogel, which can be deconvoluted into four peaks. The peaks at 530.2 eV and 531.4 eV are assigned to Fe–O and C–O–Fe accordingly,51 suggesting the linkage of α-Fe with porous carbon through this bond, which increased the stable of the adsorbent in the recycles. In addition, the peak at 532.3 eV is attributed to the Fe–OH or C–O–C,51,52 and the peak at 533.5 eV is ascribed to C–OH and/or C–O–C groups.52 The presence of oxygen functional groups could be due to the incomplete carbonization of the Fe-based cotton fibers or part formation of iron oxide during pyrolysis process, but the survey spectrum shows the intensity of oxygen element is very weak. The characteristic peak at 707.6 eV (ref. 53) can be assigned to the α-Fe of MCF aerogel. In addition, the densities and distributions of the C and Fe elements in MCF aerogel were evaluated using XPS microarea mapping. Carbon and iron are homogeneously distributed over the entire surface of MCF aerogel (Fig. 5f), which is consistent with the TEM and EDX results.
C/C–C (284.5 eV) and sp3 C–C/C–O (285.5 eV).50,51 Fig. 5c shows the O1s spectrum of MCF aerogel, which can be deconvoluted into four peaks. The peaks at 530.2 eV and 531.4 eV are assigned to Fe–O and C–O–Fe accordingly,51 suggesting the linkage of α-Fe with porous carbon through this bond, which increased the stable of the adsorbent in the recycles. In addition, the peak at 532.3 eV is attributed to the Fe–OH or C–O–C,51,52 and the peak at 533.5 eV is ascribed to C–OH and/or C–O–C groups.52 The presence of oxygen functional groups could be due to the incomplete carbonization of the Fe-based cotton fibers or part formation of iron oxide during pyrolysis process, but the survey spectrum shows the intensity of oxygen element is very weak. The characteristic peak at 707.6 eV (ref. 53) can be assigned to the α-Fe of MCF aerogel. In addition, the densities and distributions of the C and Fe elements in MCF aerogel were evaluated using XPS microarea mapping. Carbon and iron are homogeneously distributed over the entire surface of MCF aerogel (Fig. 5f), which is consistent with the TEM and EDX results.
Nitrogen sorption isotherm (Fig. 6a) of the MCF aerogel exhibits type-IV curves with a sharp capillary condensation step in the relative pressure range of 0.4–0.9 and H1-type hysteresis loop, indicative of developed mesopore.54 However, the nitrogen isotherm of the DCC aerogel shows type I curves, which present a characteristic of microporous material. Results show that the BET surface area and total pore volume of the MCF aerogel (353 m2 g−1 and 0.25 cm3 g−1, respectively) sample are higher than that of DCC (176 m2 g−1 and 0.14 cm3 g−1, respectively). The average mesoporous size of MCF aerogel (4.3 nm) is obviously higher than DCC (2.0 nm) in the large nanoscale range, which may be beneficial for the improvement in oil/solvents absorption rate under capillary action. Additionally, the fibers are intertwined together as evidenced by the SEM micrographs, thus forming macropores whose sizes cannot be determined reliably by nitrogen sorption. In short, the MCF aerogel with hierarchical porous texture is very beneficial for oil-cleanup.
A plausible formation process was proposed. The abundant –OH groups in cotton showed strong interaction with the Fe3+ ions, so some Fe3+ ions were adsorbed by the cotton to form an cotton-Fe precursor. Fe loaded on the biomass in the form of FeCl3 was hydrolyzed to Fe hydroxides (FeO(OH)) in the drying process. Some Fe hydroxides (FeO(OH)) were formed directly due to the hydrolyzation of the ferric iron. These Fe hydroxides were wrapped by the cotton. Finally, the Fe species were uniformly dispersed in the cotton matrix. The thermal decomposition of the precursors at 800 °C for 3 h was totally different. In the case of FeCl3, the iron salts were decomposed to form Fe3O4 under mesothermal conditions at 400–600 °C. Fe3C gradually formed and was subsequently transformed into Fe (600–700 °C). Some carbon atoms were incorporated into the iron oxide phase to form dense Fe3C layers, and Fe3C gradually formed and was subsequently transformed into Fe (700 °C), which was important for the formation of the sheet-like carbon. When the carbonization temperatures further increased (800 °C), the active carbon atoms in the Fe3C layers could diffuse out to form dense 2D carbon-atom layers (sheets) on the surfaces of the formed planar “Fe-template” layers, and an excess amount of carbon atoms were deposited on the formed sheets. The formed Fe3C phase acted as a template to limit the carbon graphitization along the 2D plane. In this formation process, the Fe catalyst favored the formation of a dense layer-like “Fe-template”, that limited the growth of the carbon atoms along the 2D plane.35b Meanwhile, the associated gas acted as another pore-forming agent, resulting in further improvement of the porosity during pyrolysis.
The magnetic property of the MCF aerogel was measured at room temperature. Result shows that the MCF aerogel has a saturation magnetization of 45.6 emu g−1 (Fig. 6c), which is sufficient for separation by an external magnetic field (Fig. 6d).
3.2 Application of MCF aerogel for oil–water separation
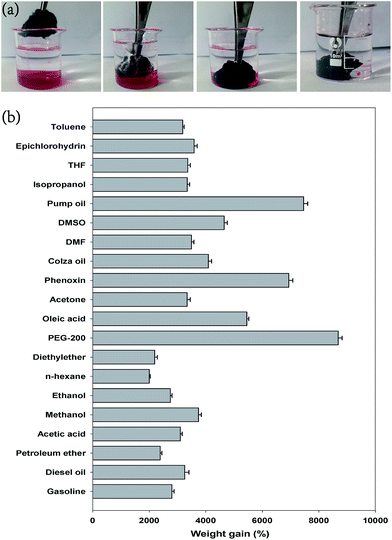
The MCF aerogel with low apparent density, fine mechanical property, high porosity and surface hydrophobicity–superoleophilicity can be used as an ideal candidate for the sorption of oils and other organic pollutants. When the MCF aerogel was brought into contact with n-hexane layer (stained with Sudan III) on a water surface, the n-hexane layer was completely and rapidly absorbed within 30 s (Fig. S10†). In particular, the MCF aerogel floated on the water surface after sorption of the n-hexane due to its low density and hydrophobicity, indicating its potential use for the facile removal of oils and other organic pollutants. In addition, the MCF aerogel can also be used to quickly absorb phenoxin (also dyed with Sudan III) under water (Fig. 7a). To further demonstrate the sorption abilities of MCF and DCC aerogels, we investigated their sorption capacities for different commercial petroleum products (e.g., gasoline, diesel oil, pump oil, etc.) and toxic organic solvents (e.g., toluene, DMSO, THF, DMF, etc.). These solvents are common pollutants in our daily life as well as from industry. In addition, the adsorption of organic solvents including ethanol and acetone was also tested. Results showed that the MCF aerogel can uptake these liquids at 22 to 87 times its own weight for all of the aforementioned organic liquids (Fig. 7b). In addition, the sorption ability of DCC aerogel (30 to 96 times) is slightly higher than the MCF aerogel, which may be ascribed to the morphological differences between MCF and DCC aerogels. Besides the above, the sorption property of MCF aerogel was typically investigated in a 1 mol L−1 NaCl aqueous solution. Results showed that the sorption ability of MCF aerogel in NaCl aqueous solution (20 to 84 times) is slightly lower than the MCF aerogel in water (22 to 87 times).Interestingly, the as-obtained MCF aerogel has showed much higher sorption capacity than many previously reported sorbents (Table S1†), such as marshmallow-like macroporous gels (6–15 times),1 wool-based nonwoven (9–15 times),13 polymers (5–25 times),55 nanowire membrane (4–20 times),20 graphene/α-FeOOH aerogel (10–30 times),56 magnetic exfoliated graphite (30–50 times);57 they are comparable to those of spongy graphene (20–86 times)3 and CNT sponge doped with boron (25–125 times),7 but lower than those of ultralight graphene framework (200–600 times),58 carbon nanotube sponges (80–180 times),25 and ultra-flyweight CNT/graphene hybrid aerogels (215–743 times).59
Since mass-based absorption capacity is strongly affected by the density of oils and absorbing materials,31,37 the volume-based absorption capacity (Voil/VMCF) was further used for investigating the absorptive capacity of the MCF aerogel. The volume absorption capacities of the present aerogel for oils and organic solvents are higher than 130% and 106% accordingly (Fig. S11†), indicating that the whole volume of MCF aerogel is used for organic liquids storage. These as-obtained values are higher than those of high-capacity carbon-based materials including CNT sponges (70–140% for hexane and 60–120% for chloroform),25 nanocellulose aerogels (80% for hexane and 70% for chloroform),37 and N-doped graphene frameworks (78.1% for gasoline),58 but are far higher than carbon ultralightweight aerogels (25.6% for crude oil and 5.9% for toluene).59 Although the sorption capacity of MCF aerogel is still lower than those of ultralight graphene framework, ultra-flyweight CNT/graphene hybrid aerogels, the production method for MCF aerogel is simplest and its precursor material is the cheapest among all these sorbents. Therefore, our MCF aerogel is a cost-effective and promising sorbent for the removal of pollutants. Based on the sorption capacities of all organic liquids in Fig. 7a and their densities, we calculated pore volumes of 30–86 cm3 g−1 for MCF aerogel (see Table S2†), in good agreement with the value of ca. 79 cm3 g−1 calculated from the apparent density.
Buoyancy is also an important parameter of oil sorbent for practical operation in oil spill cleanup over water. High buoyancy can keep the sorbent floating over the water surface before and after the oil sorption, which is helpful for the oil sorption and remove from the spilled area. In a static system, when placing MCF aerogel on the surface of oil over the water, the sorbent floats on the surface and keeps sucking the oil. After 30 min, the MCF aerogel could absorb all pump oil and still float on the water. In dynamic systems, the test is carried out under constant steering (approximately 500 rpm) in a magnetic stirrer. The MCF aerogel also has good buoyancy and high sorption capacity in dynamic systems. The high buoyancy is due to the MCF aerogel has low density and oleophilic hydrophobic properties.
3.3 Kinetics of MCF aerogel for oil–water separation
The sorption kinetics of MCF aerogel to four organic liquids was investigated. The sorption capacity Qt of each organic liquid was plotted as a function of the sorption time (Fig. S12†). It can be obviously seen that the sorption capacities increase with sorption time until saturation. Results also showed that the saturation sorption time of low-viscosity organic solvents (ethanol and phenoxin are less that 30 s accordingly) is much shorter than that of high-viscosity oils (colza oil and diesel oil are more than 800 s accordingly). This phenomenon can be ascribed to the fact that the low-viscosity organic solvents penetrate the porous network structure of MCF aerogel much more easily under capillary action. The capillary force is related to the pore size and structure, the properties of the oil and the wetting of the MCF aerogel by the organic liquids. Therefore, for different oils or solvents, the capillary force is different, resulting in different sorption and diffusion rates.60 The sorption kinetics process can be described by the second-order model:7
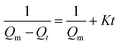 | (1) |
The mechanism of oil sorption by sorbents can be adsorption, absorption, capillary action, or a combination of these.61 For fibrous porous sorbent, the adsorption and capillary action are the main controlling mechanism. Therefore, many parameters, such as the fiber properties (fiber diameter, surface configuration, lipophilicity, special surface area, density, etc.), the oil properties (special gravity, viscosity), pore structures in sorbent (porosity, pore sizes, pore shape, etc.), as well as the interactions between oils with sorbents affect the oil sorption capacity. Some articles reported on the influences of fiber properties (pore structures), oil properties, and oil/sorbent interaction on the oil sorption capacity.61 Here, the sorption ability of MCF aerogel was investigated in an aqueous solution with pH values ranging from 3–13. Results showed that the oil–water separation efficiency was slightly varied under the above-mentioned pH values. It could be ascribed to the surface charge distribution of MCF was improved in the scope of the test. More detailed works are needed in the future. Overall, the organics were mainly stored in the macropores of the MCF aerogel, so the differences of adsorption capacities were related to the densities and viscosities of organic liquids and surface charge distribution except above-mentioned factors.
3.4 Reusability of MCF aerogel
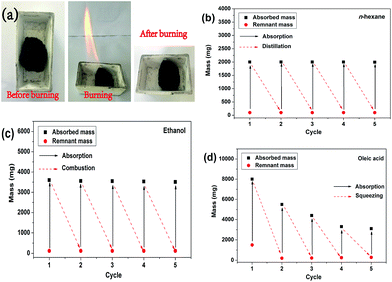
Recyclability of the MCF aerogel and recoverability of oils and organic solvents are key requirements in practical oil cleanup applications.7 The recycling process of MCF aerogel is showed in Fig. 8. For instance, combustion can remove the adsorbed ethanol by MCF aerogel (Fig. 8a). In contrast, oleic acid with a high boiling point of 350–360 °C can be recovered by squeezing the aerogel. Moreover, reproducible tests were performed for MCF aerogel through distillation (Fig. 8b), combustion (Fig. 8c) and squeezing (Fig. 8d).To testify the cyclic distillation test, the MCF aerogel was used for adsorption of n-hexane with a boiling point of 69 °C. Less than 1 wt% of residual n-hexane remained in the MCF aerogel after each cycle, and no obvious change of adsorption capacity was observed after five cycles (Fig. 8c), indicating a jarless adsorption and recycling property of the MCF aerogel. In addition, no clear structural damage was observed from the MCF fibers after the test (Fig. S13†). Ethanol was used as the absorbate for recycle of combustion. After five cycles of adsorption–combustion process, the adsorption capacity of the MCF aerogel dropped by 2.5% compared to the capacity in the first cycle (Fig. 8c), which could be ascribed to the deposition of residues on the surface of fibers after combustion of ethanol (Fig. S14†). Especially, the fibrous networks and twisted structure of the MCF aerogel remained after the repeated adsorption–combustion cycles (Fig. S14†). Finally, the higher boiling point or being nonflammable pollutants could be recovered by the cyclic adsorption-squeezing test.4 In the first cycle, 769 mg of oleic acid with a high boiling point of 350–360 °C as an example could be sorbed by the MCF aerogel, but the remnant mass became up to 293 mg after squeezing, which can be attributed to the incomplete compression of the MCF aerogel. The MCF aerogel can keep the well adsorption capability if the strain caused by squeezing should not exceed 80%, which is in accordance with the carbon fiber aerogel made from raw cotton.4 Otherwise, the long twisted carbon fibers could be broken into many short fibers (Fig. S15†). Compared with the squeezing method, the distillation method is recommendable strategy for recycling the pollutants in a reasonable range of boiling point (Fig. 9). Moreover, the MCF aerogel still kept highly hydrophobic/oleophilic characteristics after repeatedly removing oils or organics from water for 5 cycles.
3.5 Operating conditions and isolation of MCF aerogel
Usually, the sorbents were used for disposing the oil leakage accidents under ambient conditions, it is hope for materials working effectively under harsh conditions. Generally speaking, traditional sorbents including polyurethane-based and polyethylene-based materials cannot be used at temperatures above 200 °C, and others will become very brittle at low temperatures.7 In contrast, the MCF aerogel exhibits exceptional features in this respect. The MCF aerogel did not support any burning and remained inert when it exposed to an ethanol flame (data was not shown). In addition, the MCF aerogel was immediately immersed into liquid nitrogen after ca. 1 min of such extreme heating. Results showed that the shape, volume, and inherent porous structure of MCF aerogel were still remained unchanged after several times treatment, indicating that the MCF aerogel can withstand extreme temperatures and rapid temperature change, which further validated the TGA results (Fig. S9†). No obvious effect on the hydrophobic and oleophilic properties of the MCF aerogel was observed after the high and low temperature treatments. Meanwhile, the MCF aerogel retains its hydrophobicity for at least one year under atmospheric conditions, manifesting the MCF aerogel can be used under the demanding conditions found in oil recovery.Interestingly, the MCF aerogel could also be easily driven to the oil-polluted region by a magnet bar because of its magnetic property, providing a facile and energy-saving method to collect oils from a polluted water area.31 In particular, the MCF aerogel still kept favourable separation efficiency in an aqueous solution with pH values ranging from 3–13 for 18 h. These interesting and important properties make the MCF aerogel as a kind of promising oil-absorbent material for quickly cleaning large-area oil spills. Compared with the existing high-capacity absorptive materials including magnetic foams,31 graphene and CNT foams,25,58,59 the MCF aerogel was fabricated through a facile procedure using cheap cottons, which is suitable for large-scale production.
4. Conclusion
In summary, a facile method was successfully used for fabricating the MCF aerogel by one-step pyrolysis of FeCl3-coated cotton. The MCF aerogel exhibited high hydrophobicity/superoleophilicity property without the modification of low-surface-energy chemicals, especially it had good thermal stability and mechanical properties, high adsorption capacity of 22–87 times its own weight, and superior separation efficiency. The distillation, combustion and squeezing processes can be used for the recycling of the MCF aerogel. Most importantly, the natural material and facile fabrication method make the MCF aerogel cost-effective for oil–water separation. Therefore, the MCF aerogel is highly promising as a low-cost, potential, and environmentally friendly sorbent for environmental and ocean protection. In addition, it is also anticipated that MCF aerogel can be used as electrode material for energy storage devices, and magnetic adsorbent for pre-concentration of target compounds, as well as a building block for functional composite material.Acknowledgements
This work was financially supported by the National Natural Science Foundations of China (Grant No. 81173024, 81227802, and 21275098).Notes and references
- G. Hayase, K. Kanamori, M. Fukuchi, H. Kaji and K. Nakanishi, Angew. Chem., Int. Ed., 2013, 52, 1986–1989 CrossRef CAS PubMed.
- A. V. Dudchenko, J. Rolf, L. Shi, L. Olivas, W. Duan and D. Jassby, ACS Nano, 2015, 9, 9930–9941 CrossRef CAS PubMed.
- H. C. Bi, X. Xie, K. B. Yin, Y. L. Zhou, S. Wan, L. B. He, F. Xu, F. Banhart, L. Sun and R. S. Ruoff, Adv. Funct. Mater., 2012, 22, 4421–4425 CrossRef CAS.
- H. Bi, Z. Yin, X. Cao, X. Xie, C. Tan, X. Huang, B. Chen, F. Chen, Q. Yang, X. Bu, X. Lu, L. Sun and H. Zhang, Adv. Mater., 2013, 25, 5916–5921 CrossRef CAS PubMed.
- J. Lin, F. Tian, Y. Shang, F. Wang, B. Ding and J. Yu, Nanoscale, 2012, 4, 5316–5320 RSC.
- J. Lin, F. Tian, Y. Shang, F. Wang, B. Ding, J. Yu and Z. Guo, Nanoscale, 2013, 5, 2745–2755 RSC.
- Z.-Y. Wu, C. Li, H.-W. Liang, Y.-N. Zhang, X. Wang, J.-F. Chen and S.-H. Yu, Sci. Rep., 2014, 4, 4079 Search PubMed.
- M. O. Adebajo, R. L. Frost, J. T. Kloprogge, O. Carmody and S. Kokot, J. Porous Mater., 2003, 10, 159–170 CrossRef CAS.
- D. N. H. Tran, S. Kabiri, T. R. Sim and D. Losic, Environ. Sci.: Water Res. Technol., 2015, 1, 298–305 CAS.
- S. Cappello, R. Denaro, M. Genovese, L. Giuliano and M. M. Yakimov, Microbiol. Res., 2006, 162, 185–190 CrossRef PubMed.
- B. Wang, W. Liang, Z. Guo and W. Liu, Chem. Soc. Rev., 2015, 44, 336–361 RSC.
- M. Inagaki, A. Kawahara and H. Konno, Carbon, 2002, 40, 105–111 CrossRef CAS.
- M. M. Radetic, D. M. Jocic, P. M. Jovanccic, Z. L. Petrovic and H. F. Thomas, Environ. Sci. Technol., 2003, 37, 1008–1012 CrossRef CAS PubMed.
- M. Hussein, A. A. Amer and I. I. Sawsan, J. Anal. Appl. Pyrolysis, 2008, 82, 205–211 CrossRef CAS.
- T. R. Annunciado, T. H. D. Sydenstricker and S. C. Amico, Mar. Pollut. Bull., 2005, 50, 1340–1346 CrossRef CAS PubMed.
- J. Zhang and S. Seeger, Adv. Funct. Mater., 2011, 21, 4699–4704 CrossRef CAS.
- Q. Zhu, Y. Chu, Z. Wang, N. Chen, L. Lin, F. Liu and Q. Pan, J. Mater. Chem. A, 2013, 1, 5386–5393 CAS.
- P. Calcagnile, D. Fragouli, I. S. Bayer, G. C. Anyfantis, L. Martiradonna, P. D. Cozzoli, R. Cingolani and A. Athanassiou, ACS Nano, 2012, 6, 5413–5419 CrossRef CAS PubMed.
- D. Ceylan, S. Dogu, B. Karacik, S. D. Yakan, O. S. Okay and O. Okay, Environ. Sci. Technol., 2009, 43, 3846–3852 CrossRef CAS PubMed.
- J. Yuan, X. Liu, O. Akbulut, J. Hu, S. L. Suib, J. Kong and F. Stellacci, Nat. Nanotechnol., 2008, 3, 332–336 CrossRef CAS PubMed.
- S.-J. Choi, T.-H. Kwon, H. Im, D.-I. Moon, D. J. Baek, M.-L. Seol, J. P. Duarte and Y. K. Choi, ACS Appl. Mater. Interfaces, 2011, 3, 4552–4556 CAS.
- B. Chen, Q. Ma, C. Tan, T. T. Lim, L. Huang and H. Zhang, Small, 2015, 11, 3319–3336 CrossRef CAS PubMed.
- S. Nardecchia, D. Carriazo, M. L. Ferrer, M. C. Gutierrez and F. del Monte, Chem. Soc. Rev., 2013, 42, 794–830 RSC.
- R. W. Fu, B. Zheng, J. Liu, M. S. Dresselhaus, G. Dresselhaus, J. H. Satcher Jr and T. F. Baumann, Adv. Funct. Mater., 2003, 13(7), 558–562 CrossRef CAS.
- X. C. Gui, J. Q. Wei, K. L. Wang, A. Y. Cao, H. W. Zhu, Y. Jia, Q. Shu and D. Wu, Adv. Mater., 2010, 22, 617–621 CrossRef CAS PubMed.
- M. K. Shi, J. Oh, M. Lima, M. E. Kozlov, S. J. Kim and R. H. Baughman, Adv. Mater., 2010, 22, 2663–2667 CrossRef PubMed.
- H. C. Bi, K. B. Yin, X. Xie, Y. L. Zhou, N. Wan, F. Xu, F. Banhart, L. Sun and R. S. Ruoff, Adv. Mater., 2012, 24, 5124–5129 CrossRef CAS PubMed.
- Z.-Y. Wu, C. Li, H.-W. Liang, J.-F. Chen and S.-H. Yu, Angew. Chem., Int. Ed., 2013, 52, 2925–2929 CrossRef CAS PubMed.
- H.-W. Liang, Q.-F. Guan, L.-F. Chen, Z. Zhu, W.-J. Zhang and S.-H. Yu, Angew. Chem., Int. Ed., 2012, 51, 5101–5105 CrossRef CAS PubMed.
- H.-W. Liang, J.-W. Liu, H.-S. Qian and S.-H. Yu, Acc. Chem. Res., 2013, 46, 1450–1461 CrossRef CAS PubMed.
- N. Chen and Q. Pan, ACS Nano, 2013, 7(8), 6875–6883 CrossRef CAS PubMed.
- L. Wu, L. Li, B. Li, J. Zhang and A. Wang, ACS Appl. Mater. Interfaces, 2015, 7(8), 4936–4946 CAS.
- A. Turco, C. Malitesta, G. Barillaro, A. Greco, A. Maffezzoli and E. Mazzotta, J. Mater. Chem. A, 2015, 3, 17685–17696 CAS.
- D. L. Sivadas, R. Narasimman, R. Rajeev, K. Prabhakaran and K. N. Ninan, J. Mater. Chem. A, 2015, 3, 16213–16221 CAS.
- (a) Z. Wen, S. Ci, F. Zhang, X. Feng, S. Cui, S. Mao, S. Luo, Z. He and J. Chen, Adv. Mater., 2012, 24, 1399–1404 CrossRef CAS PubMed; (b) S. Zhang, M. Zeng, J. Li, J. Li, J. Xu and X. Wang, J. Mater. Chem. A, 2014, 2, 4391–4397 RSC.
- Z. Wang, Y. Xu, Y. Liu and L. Shao, J. Mater. Chem. A, 2015, 3, 12171–12178 CAS.
- J. T. Korhonen, M. Kettunen, R. H. A. Ras and O. Ikkala, ACS Appl. Mater. Interfaces, 2011, 3, 1813–1816 CAS.
- G. Ghosha, A. Vilcheza, J. Esquenaa, C. Solansa and C. Rodriguez-Abreu, Mater. Chem. Phys., 2011, 130, 786–793 CrossRef.
- W. F. Chen, S. R. Li, C. H. Chen and L. F. Yan, Adv. Mater., 2011, 23, 5679–5683 CrossRef CAS PubMed.
- M. Popovici, M. Gich and C. Savii, Rom. Rep. Phys., 2006, 58, 369–378 CAS.
- M. Gich, L. Casas, A. Roig and E. Molins, Appl. Phys. Lett., 2003, 82, 4307–4309 CrossRef CAS.
- T. Li, H. Liu, L. Zeng, S. Yang, Z. Li, J. Zhang and X. Zhou, J. Mater. Chem., 2011, 21, 12865–12872 RSC.
- G. Vialle, M. D. Prima, E. Hocking, K. Gall, H. Garmestani, T. Sanderson and S. C. Arzberger, Smart Mater. Struct., 2009, 18, 115014 CrossRef.
- L. Biasetto, A. Francis, P. Palade, G. Principi and P. Colombo, J. Mater. Sci., 2008, 43, 4119–4126 CrossRef CAS.
- L. Sorrentino, M. Aurilia, G. Forte and S. Iannace, J. Appl. Polym. Sci., 2011, 119, 1239–1247 CrossRef CAS.
- S. Liu, Q. Yan, D. Tao, T. Yu and X. Liu, Carbohydr. Polym., 2012, 89, 551–557 CrossRef CAS PubMed.
- R.-L. Liu, W.-J. Ji, T. He, Z.-Q. Zhang, J. Zhang and F.-Q. Dang, Carbon, 2014, 76, 84–95 CrossRef CAS.
- Y. Liu, J. X. Xue, T. Zheng and J. R. Dahn, Carbon, 1996, 34, 193–200 CrossRef CAS.
- B. Zhang, J. Song, G. Yang and B. Han, Chem. Sci., 2014, 5, 4656–4660 RSC.
- S. Zhang, M. Zeng, J. Li, J. Li, J. Xu and X. Wang, J. Mater. Chem. A, 2014, 2, 4391–4397 CAS.
- L. Li, G. Zhou, Z. Weng, X.-Y. Shan, F. Li and H.-M. Cheng, Carbon, 2014, 67, 500–507 CrossRef CAS.
- M. Sevilla and A. B. Fuertes, Chem.–Eur. J., 2009, 15, 4195–4203 CrossRef CAS PubMed.
- H. J. Lee, W. Cho, E. Lim and M. Oh, Chem. Commun., 2014, 50, 5476–5479 RSC.
- J. Wei, D. Zhou, Z. Sun, Y. Deng, Y. Xia and D. Zhao, Adv. Funct. Mater., 2013, 23, 2322–2328 CrossRef CAS.
- A. Li, H. S. Sun, D. Z. Tan, W. J. Fan, S. H. Wen, X. J. Qing, G.-X. Li, S.-Y. Li and W.-Q. Deng, Energy Environ. Sci., 2011, 4, 2062–2065 CAS.
- H. P. Cong, X. C. Ren, P. Wang and S. H. Yu, ACS Nano, 2012, 6, 2693–2703 CrossRef CAS PubMed.
- G. Wang, Q. Sun, Y. Zhang, J. Fan and L. Ma, Desalination, 2010, 263, 183–188 CrossRef CAS.
- Y. Zhao, C. Hu, Y. Hu, H. Cheng, G. Shi and L. Qu, Angew. Chem., Int. Ed., 2012, 51, 11371–11375 CrossRef CAS PubMed.
- H. Sun, Z. Xu and C. Gao, Adv. Mater., 2013, 25, 2554–2560 CrossRef CAS PubMed.
- X. C. Gui, Z. P. Zeng, Z. Q. Lin, Q. M. Gan, R. Xiang, Y. Zhu, A. Cao and Z. Tang, ACS Appl. Mater. Interfaces, 2013, 5, 5845–5850 CAS.
- H. Zhu, S. Qiu, W. Jiang, D. Wu and C. Zhang, Environ. Sci. Technol., 2011, 45, 4527–4531 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra02794f |
| This journal is © The Royal Society of Chemistry 2016 |