Migration behavior of anionic polyurethane dispersion during infiltration and redistribution in sand
Abstract
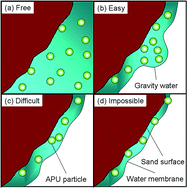
Migration behavior strongly affects the properties of the chemical sand stabilization (CSS) crust by determining the distribution of the stabilizing material in sand. A simple method was established to investigate the migration behavior of anionic polyurethane (APU) dispersion (APUD) in sand, based on weight measurement. The movements of APU particles and water were found to differentiate during the redistribution, as a result of the size restriction of film water. By analyzing the interaction between the sand surface, APU particles, and water, four patterns of the migration of APU particles were illustrated. Factors that might affect the migration, such as temperature, APU particle size, and concentration of APUD, were investigated. A semi-empirical formula was deduced for the thickness of the CSS crust, which demonstrates the relationship among sand, water, and the stabilizing material.

 Please wait while we load your content...
Please wait while we load your content...