A telescopic one-pot synthesis of β-lactam rings using amines as a convenient source of imines†
Suvi H. M. Rajamäkia,
Lidia De Lucaa,
Francesca Capittaa and
Andrea Porcheddu*b
aUniversità degli Studi di Sassari, Dipartimento di Chimica e Farmacia, via Vienna 2, Sassari, Italy
bUniversità degli Studi di Cagliari, Dipartimento di Scienze Chimiche e Geologiche, Cittadella Universitaria, SS 554 bivio per Sestu, 09042 Monserrato, Ca, Italy. E-mail: porcheddu@unica.it
First published on 14th April 2016
Abstract
A facile synthetic approach to substituted β-lactams was designed, using secondary benzylic amines and acid chlorides as starting materials. The reactions proceeded smoothly and all the products were obtained in good yields.
Since the introduction of penicillin in the 1940s,1 antibiotics have become a milestone of modern medicine.2 Penicillins, cephalosporins and monobactams all belong to the family of β-lactam antibiotics,3 in which the β-lactam ring is part of the core structure.4 Antibiotics now face a critical and unprecedented challenge resulting from the combination of two different problems. First, antibiotic resistance to commonly used drugs is increasing at an alarming rate.5 Second, the development of new antibiotics has become so expensive and unattractive for pharmaceutical companies that the antibiotic pipeline is running dry.6 The need to overcome the problem caused by drug-resistant bacteria7 has motivated chemists to modify the structure of β-lactam antibiotics, aiming to render it insensitive to the β-lactamase attack.8
Today, despite an enormous number of synthetic strategies to prepare the β-lactam ring,9 the Staudinger approach10 using ketenes and imines is regarded as the most effective and versatile procedure for the synthesis of 2-azetidinone rings (Scheme 1).11 However, the preparation of labile imines12 requires an additional synthetic step, and several issues affect the yield due to the existence of equilibrium conditions between the reactants and the corresponding imines. Taking these factors into consideration, we planned to generate imines by oxidation of the corresponding amines in order to develop an effective procedure to facilitate the access to substituted β-lactam rings.13
Following our previous work on both β-lactams14 and chloramines,15 we designed a modified Staudinger β-lactam synthesis using N-chloramines as a convenient source of imines (Scheme 1). The in situ-generated imine may subsequently react with a preformed ketene via a [2 + 2] cycloaddition to afford the corresponding β-lactam.
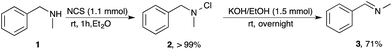
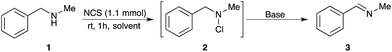
The first step was the formation of a chloramine, followed by subsequent elimination with base to form the corresponding imine. N-Benzylamine 1 (1.0 mmol), chosen as a model substrate, was dissolved in dry Et2O and treated with N-chlorosuccinimide (NCS, 1.1 mmol) at room temperature in an inert atmosphere. After 1 h, TLC analysis showed complete consumption of the starting amine, and the aqueous work-up of the reaction mixture afforded chloramine 2 in an almost quantitative yield (Scheme 2).16
In the following step, the resulting chloramine 2 (1 mmol) dissolved in Et2O was treated overnight at room temperature with an ethanolic solution of KOH (1.1 mmol), to afford the corresponding imine 3 in good yield (71%, Scheme 2).
A set of bases in different solvents was then screened to optimise the yield of imine 3 without isolating the intermediate chloramine 2.17 The best results were obtained by performing both the formation of chloramine and the elimination reaction to give the imine in sequence in acetonitrile, using triethylamine (1.5 mmol) as base (Table 1, entry 3). The imine formation was complete in just 3 hours at room temperature.
| Entry | Base | Solvent | Yielda (%) |
|---|---|---|---|
| a Yields were determined by NMR from crude reaction mixture.b DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene.c Anhydrous K2CO3.d DABCO = 1,4-diazabicyclo[2.2.2]octane.e TMG = 1,1,3,3-tetramethylguanidine.f DMAP = 4-dimethylaminopyridine. | |||
| 1 | DBUb | CH2Cl2 | 49 |
| 2 | DBU | CH3CN | 54 |
| 3 | NEt3 | CH3CN | 99 |
| 4 | NEt3 | CH2Cl2 | 85 |
| 5 | NEt3 | Et2O | 81 |
| 6 | K2CO3c | CH3CN | 8 |
| 7 | DABCOd | CH3CN | — |
| 8 | TMGe | CH3CN | — |
| 9 | NEt3/DMAPf | CH3CN | 61 |
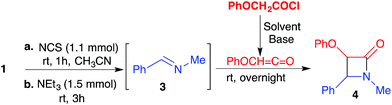
Having established a reliable and easy procedure to convert amines into imines, we then turned our attention to study the [2 + 2] cycloaddition reaction of in situ-generated imines with ketenes.
Initially, to the imine 3 was added a solution of phenoxyacetyl chloride that had been previously treated with an equimolar amount of triethylamine to generate the corresponding ketene. The pre-formed ketene reacted smoothly with the imine 3 giving a cis-β-lactam as the product (see Scheme in Table 2). In order to find the best route to prepare 2-azetidinone rings, we carried out several experiments in parallel, reacting amine 1 with various amounts of phenoxyacetyl chloride and triethylamine, as summarised in Table 2. The best results were achieved dissolving the phenoxyacetyl chloride in acetonitrile, and using 2 equivalents of acid chloride and triethylamine for each equivalent of N-methylbenzylamine (Table 2, entry 4). Once it was established that in situ imine and ketene formation, as well as the subsequent 2 + 2 rearrangement, proceeded smoothly in acetonitrile, we then explored the possibility of developing the synthesis of β-lactams 4 via a one-pot two-step protocol. Therefore, N-methylbenzylamine 1 (1 mmol) dissolved in dry acetonitrile (10 ml) was first chlorinated with NCS (1.1 mmol), treated with excess triethylamine (3.5 mmol) for 1 h under argon at rt, and finally reacted overnight with phenoxyacetyl chloride (2 mmol) in dry acetonitrile (10 ml).18 Under these optimised reaction conditions, the cis-β-lactam 4 was isolated (85%) as the major product.19
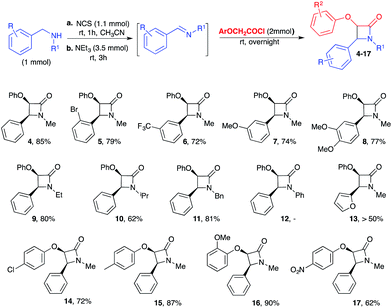
With these optimised reaction conditions, the scope and limitations of this protocol were studied applying it to various benzylic amines (Scheme 3, β-lactams 5–14). Benzylic amines with either an electron-withdrawing (2-Br, 3-CF3) or an electron-donating group (3-MeO or 3-MeO, 4-MeO) on the aryl ring proved to be suitable substrates for this reaction, affording the corresponding β-lactams 5–8 in high yields. The position and activating or deactivating nature of substituents on the rings did not significantly affect the diastereoselectivity of the reaction (cis/trans > 9/1) and they also had negligible influence on the yield of the isolated products (ranging from 72% to 79%).
 | ||
| Scheme 3 Synthesis of α-phenyloxy-β-lactams with aryloxyacetyl chlorides and an array of benzyl amine derivatives. | ||
Ethyl, isopropyl and benzyl groups on the benzylic nitrogen were well tolerated, providing the corresponding β-lactams 9–11 in high overall isolated yields. Conversely, the reaction with N-benzyl anilines (Scheme 3, β-lactam 12) was problematic and only traces of product were detected.20
In the case of β-lactam 13, the product was formed in good yield as judged by 1H NMR analysis of a sample of the crude reaction mixture. Unfortunately the presence of many side products rendered the reaction unviable and any attempt to purify the product proved unsuccessful. It is worth noting that aryloxyketenes possessing a substituent (Me, OMe, Cl, NO2) on the aromatic ring (Scheme 3, β-lactams 14–17) were compatible with the designed method. Moreover, in general, acid chlorides containing electron-rich substituents on the phenyl ring provided better yields than those bearing electron-withdrawing groups (Scheme 3).
After these encouraging results from the initial experiments, the general scope of this protocol was further explored by reacting the in situ-generated imine 3 with a set of different acid chlorides used as precursors of ketenes. The reaction worked well also with methoxyacetyl chloride (Scheme 4, β-lactams 18–22). The presence of a longer side chain on the ketene did not significantly affect the final yield (Scheme 4, β-lactams 22). Although the yields of these 2-azetidinones were somewhat lower than with phenoxyacetyl chloride, they should be considered satisfactory, since reactions are performed in a one-pot, three-step fashion.
Surprisingly, when the phenoxyacetyl chloride was replaced with phenylacetyl chloride, no product was obtained when the reaction was carried out at room temperature. We reoptimised the reaction conditions and we were delighted to find that heating the reaction mixture overnight at 60 °C in the ketene formation step, the Staudinger reaction proceeded as smoothly as before, affording the β-lactam 23 in reasonable yields (Scheme 5).
The reaction yields decreased slightly for substituted phenylacetyl chlorides bearing electron-withdrawing groups on the aromatic ring (Scheme 5, compare compounds 23 and 24). In contrast, electron-donating groups increased the yields significantly, giving an overall quantitative yield of cis and trans isomers (Scheme 5, see β-lactams 25 and 26). We also attempted to carry out the reaction with aliphatic acyl chlorides, but the annulation reaction to give β-lactam 27 was unsuccessful (Scheme 5).21
It is important to note that the reaction with (phenylthio)acetyl chloride failed to give the desired β-lactam 28 at room temperature, but with the procedure used for phenylacetyl chlorides the reaction proceeded smoothly and in good yield, giving the trans isomer as the only isolated product.
Next, we decided to explore the scope of this modified Staudinger procedure combining the ketene derived from the phenylacetyl chloride with a set of imines generated from substituted benzylic amines. Generally, substituents on the aryl group of N-methylbenzylamine do not seem to influence the outcome of the reaction (Scheme 5, β-lactams 29 and 30). When N-methylfurfurylamine was employed, product 31 was identified in the crude NMR, but as for β-lactam 13, isolation and purification proved very difficult and the product was not isolated in acceptable purity.
The reaction failed to provide the desired β-lactam using aliphatic amines with either of the protocols. The reaction was tested with N-methylbutylamine and phenoxyacetyl chloride, varying the base22 used in imine formation as well as solvent and temperature of the reaction (Scheme 6).
While the synthesis of the chloramine proceeded smoothly, both imine formation and the subsequent Staudinger reaction with ketene to give a β-lactam resulted in a complex mixture of products, unidentifiable by NMR.
Despite some unsuccessful attempts, we were pleasantly surprised by the high number of unknown compounds (>85%)23 prepared using this procedure, suggesting that our methodology may provide a direct and alternative route to β-lactam structures.
On the basis of above results and previously published mechanisms,24 we postulate the most plausible reaction pathway for the overall conversion of secondary benzyl amines and acid chlorides into β-lactams (Scheme 7). In the first step, NCS promotes the chlorination of the N-alkylamine 32, releasing an equivalent amount of succinimmide. In the second step, NEt3 abstracts a proton from both chloramine 33 and acid chloride 34 generating the imine 35 and ketene 36 respectively. In the following step, the imine nitrogen attacks the ketene giving a zwitterionic intermediate, 37. The subsequent intramolecular ring closure of the zwitterionic intermediate delivers β-lactam 38. The cis or trans configuration of the final β-lactam is always the result of a complex dynamic balance between two competitive reactions involving the conrotatory ring closure on one hand and the imine isomerization (39) of the zwitterionic intermediate 37 on the other.
 | ||
| Scheme 7 A plausible reaction pathway for the overall conversion of secondary benzyl amines and acid chlorides into β-lactams. | ||
In general, electron-donating (EDG) ketene substituents accelerate direct ring closure, promoting the formation of cis-β-lactam 38. On the contrary, weak electron-donating and electron-withdrawing (EWG) ketene substituents slow down direct ring closure, strongly favouring trans-β-lactam isomer.
Conclusions
A facile synthetic approach to substituted β-lactams was designed, starting from secondary benzyl amines and acid chlorides. Instead of classical imines, which are labile molecules, secondary amines are used expanding the molecular diversity of the traditional Staudinger methodology. Generally, the reactions proceeded smoothly and all synthesised products were isolated in good to high yields. This modification of the traditional Staudinger synthesis, where both imine and ketene are formed in situ from cheap starting materials and no additives apart from a base are used, reduces both the costs and the purification steps of the procedure to a minimum.Acknowledgements
Financial support from the Università degli Studi di Cagliari (PRID 2015) and the Regione Autonoma della Sardegna (Project L.R. 7/2007, annualità 2012, CUP F71J120001090002) is gratefully acknowledged. We are also grateful to Fondazione Banco di Sardegna (Project: Prot. U875.2014/AI.758.MGB, Prat.2014.0803).Notes and references
- American Chemical Society International Historic Chemical Landmarks. Discovery and Development of Penicillin. Retrieved from http://www.acs.org/content/acs/en/education/whatischemistry/landmarks/flemingpenicillin.html.
- Antibiotics have not only saved millions of human lives, but they have opened up significant new vistas for research that would have been unmanageable without them. L. Demain and R. P. ElanderAntonie van Leeuwenhoek 1999, 75 5–19 CrossRef; W. Dürckheimer, J. Blumbach, R. Lattrell and K. H. Scheunemann, Angew. Chem., Int. Ed. Engl., 1985, 24, 180–202 CrossRef; A. Cometta and M. P. Glauser, Schweiz. Med. Wochenschr., 1988, 118, 1817–1822 Search PubMed; R. Bud, Penicillin: Triumph and Tragedy, Oxford University Press, Oxford, 2009 Search PubMed; E. Torok, E. Moran and F. Cooke, Oxford Handbook of Infectious Diseases and Microbiology, Oxford University Press, Oxford, 2009 Search PubMed.
- “List of β-lactam antibiotics”, retrieved from https://en.wikipedia.org/wiki/β-lactam_antibiotic.
- P. G. Sammes, Chem. Rev., 1976, 76, 113–155 CrossRef CAS; R. B. Morin and M. Gorman, Chemistry and Biology of β-Lactam Antibiotics, Academic Press, New York, 1982, vol. 1–3 CrossRef; W. Dürkheimer, J. Blumbach, R. Lattrell and K. H. Scheunemann, Angew. Chem., 1985, 97, 183–205 CrossRef; W. Dürkheimer, J. Blumbach, R. Lattrell and K. H. Scheunemann, Angew. Chem., Int. Ed. Engl., 1985, 24, 180–202 CrossRef; Recent Progress in the Chemical Synthesis of Antibiotics and Related Microbial Products, ed. R. Southgate, C. Branch, S. Coulton, E. Hunt and G. Lukacs, Springer-Verlag, Berlin, 1993, vol. 2, p. 621 RSC; R. Southgate, M. Jayaraman, A. R. A. S. Deshmukh and B. M. Bhawal, Contemp. Org. Synth., 1994, 1, 417–432 RSC; E. L. Setti, D. Davis, T. Chung and J. McCarter, Bioorg. Med. Chem. Lett., 2003, 13, 2051–2053 CrossRef PubMed; B. K. Banik, β-Lactams: Unique Structures of Distinction for Novel Molecules, Springer, 2013 Search PubMed.
- D. M. Shlaes, Antibiotics: The Perfect Storm, Springer, Dordrecht: Heidelberg, London, NY, USA, 2010 CrossRef CAS PubMed; J. N. Pendleton, S. P. Gorman and B. F. Gilmore, Expert Rev. Anti-Infect. Ther., 2013, 11, 297–308 CrossRef CAS PubMed; W. Qin, M. Panunzio and S. Biondi, Antibiotics, 2014, 3, 193–215 CrossRef PubMed.
- This has led to an alarming situation where some bacterial infections are difficult, if not impossible, to cure. Over time this problem looks set to get worse. D. I. Andersson and D. Hughes, Nat. Rev. Microbiol., 2010, 8, 260–271 Search PubMed; D. Jabes, Curr. Opin. Microbiol., 2011, 14, 564–569 CrossRef CAS PubMed; T. R. Frieden, Antibiotic resistance threats in the United States, Cdc, 2013, pp. 22–50 CrossRef PubMed; T. R. Frieden, N. Engl. J. Med., 2015, 373, 1748–1754 CrossRef PubMed.
- M. G. P. Page, Drug Resist. Updates, 2000, 3, 109–125 CrossRef CAS PubMed; S. Driussi, Antibiotic Resistant Bacteria, Hayle Medical, 2015 Search PubMed.
- S. M. Drawz and R. A. Bonomo, Clin. Microbiol. Rev., 2010, 23, 160–201 CrossRef CAS PubMed; D. M. Shlaes, Ann. N. Y. Acad. Sci., 2013, 1277, 105–114 CrossRef PubMed; R. R. Watkins, K. M. Papp-Wallace, S. M. Drawz and R. A. Bonomo, Front. Microbiol., 2013, 4, 1–8 Search PubMed; S. M. Drawz, K. M. Papp-Wallace and R. A. Bonomo, Antimicrob. Agents Chemother., 2014, 58, 1835–1846 CrossRef PubMed; I. Olsen, Eur. J. Clin. Microbiol. Infect. Dis., 2015, 34, 1303–1308 CrossRef PubMed.
- G. S. Singh, Tetrahedron, 2003, 59, 7631–7649 CrossRef CAS; Heterocyclic Scaffolds I: β-Lactams, ed. B. K.Banik, Springer, Heidelberg, 2009, vol. 1 Search PubMed; G. S. Singh and S. Sudheesh, ARKIVOC, 2014, 337–385 Search PubMed.
- H. Staudinger, Justus Liebigs Ann. Chem., 1907, 356, 51–123 CrossRef CAS; W.-B. Wang and E. J. Roskamp, J. Am. Chem. Soc., 1993, 115, 9417–9420 CrossRef; G. Martelli, G. Spunta and M. Pannunzio, Tetrahedron Lett., 1998, 39, 6257–6260 CrossRef; M. Pannunzio, S. Bacchi, E. Campana, L. Fiume and P. Vicennati, Tetrahedron Lett., 1999, 40, 8495–8498 CrossRef; C. M. L. Delpiccolo, M. A. Fraga and E. G. Mata, J. Comb. Chem., 2003, 5, 208–210 CrossRef PubMed; A. Weatherwax, C. J. Abraham and T. Lectka, Org. Lett., 2005, 7, 3461–3463 CrossRef PubMed; F. L. Lin, H. M. Hoyt, H. Van Halbeek, R. G. Bergman and C. R. Bertozzi, J. Am. Chem. Soc., 2005, 127, 2686–2695 CrossRef PubMed; Y. Wang, Y. Liang, L. Jiao, D. M. Du and J. Xu, J. Org. Chem., 2006, 71, 6983–6990 CrossRef PubMed; L. Jiao, Y. Liang and J. Xu, J. Am. Chem. Soc., 2006, 128, 6060–6069 CrossRef PubMed; B. Li, Y. Wang, D.-M. Du and J. Xu, J. Org. Chem., 2007, 72, 990–997 CrossRef PubMed; Y. Liang, L. Jiao, S. Zhang, Z.-X. Yu and J. Xu, J. Am. Chem. Soc., 2009, 131, 1542–1549 CrossRef PubMed.
- C. Palomo and J. M. Aizpurua, Chem. Heterocycl. Compd., 1999, 34, 1222–1236 CrossRef CAS; C. Palomo, J. M. Aizpurua, I. Ganboa and M. Oiarbide, Eur. J. Org. Chem., 1999, 3223–3235 CrossRef; A. Arrieta, F. P. Cossio and B. Lecea, J. Org. Chem., 2000, 65, 8458–8464 CrossRef PubMed; A. E. Taggi, A. M. Hafez, H. Wack, B. Young, D. Ferraris and T. Lectka, J. Am. Chem. Soc., 2002, 124, 6626–6635 CrossRef PubMed; M. Köhn and R. Breinbauer, Angew. Chem., Int. Ed., 2004, 43, 3106–3116 CrossRef PubMed; S. Eguchi, ARKIVOC, 2005, 98–119 Search PubMed; F. L. Lin, H. M. Hoyt, H. Van Halbeek, R. G. Bergman and C. R. Bertozzi, J. Am. Chem. Soc., 2005, 127, 2686–2695 CrossRef PubMed; L. Jiao, Y. Liang and J. Xu, J. Am. Chem. Soc., 2006, 128, 6060–6069 CrossRef PubMed; N. Fu and T. T. Tidwell, Tetrahedron, 2008, 64, 10465–10496 CrossRef; F. P. Cossío, A. Arrieta and M. A. Sierra, Acc. Chem. Res., 2008, 41, 925–936 CrossRef PubMed; J. Burés, M. Martín, F. Urpí and J. Vilarrasa, J. Org. Chem., 2009, 74, 2203–2206 CrossRef PubMed; Name reactions for carbocyclic ring formations, ed. J. J.Li, Wiley, Hoboken, N. J., 2010, p. 47 Search PubMed; M. Zarei and A. Jarrahpour, Synlett, 2011, 2572–2576 Search PubMed; Z. Yang and J. Xu, Tetrahedron Lett., 2012, 53, 786–789 CrossRef; I. Kim, S. W. Roh, D. G. Lee and C. Lee, Org. Lett., 2014, 16, 2482–2485 CrossRef PubMed; C. R. Pitts and T. Lectka, Chem. Rev., 2014, 114, 7930–7953 CrossRef PubMed; Z. Yang, N. Chen and J. Xu, J. Org. Chem., 2015, 80, 2–11 Search PubMed.
- A. T. Williamson, J. Am. Chem. Soc., 1934, 56, 2216–2218 CrossRef CAS; G. Friedrichs and H. G. Wagner, Z. Phys. Chem., 2001, 215, 1601–1623 CrossRef; T. T. Tidwell, Eur. J. Org. Chem., 2006, 563–576 CrossRef; A. D. Allen and T. T. Tidwell, Eur. J. Org. Chem., 2012, 1081–1096 CrossRef; W. Qin, S. Long, M. Panunzio and S. Biondi, Molecules, 2013, 18, 12264–12289 CrossRef PubMed.
- We took inspiration from nature, where aldehydes and ketones are generated in situ (via imines) by oxidation of corresponding amino groups.
- D. Donati, C. Morelli, A. Porcheddu and M. Taddei, J. Org. Chem., 2004, 69, 9316–9318 CrossRef CAS PubMed.
- L. De Luca and G. Giacomelli, Synlett, 2004, 2180–2184 CrossRef CAS; L. De Luca, G. Giacomelli and G. Nieddu, Synlett, 2005, 223–226 Search PubMed; R. Cadoni, A. Porcheddu, G. Giacomelli and L. De Luca, Org. Lett., 2012, 14, 5014–5017 CrossRef PubMed; A. Porcheddu and L. De Luca, Adv. Synth. Catal., 2012, 354, 2949–2953 CrossRef; S. Gaspa, A. Porcheddu and L. De Luca, Org. Biomol. Chem., 2013, 11, 3803–3807 Search PubMed.
- In order to avoid possible decomposition of N-chloramine 2, it was recovered by removing the extraction solvent at room temperature and reduced pressure M. R. Monaco, P. Renzi, D. M. Scarpino Schietroma and M. Bella, Org. Lett., 2011, 13, 4546–4549 CrossRef CAS PubMed and references cited therein.
- The previously optimised protocol for preparing imine 3 is not suitable for a one-pot procedure. Unfortunately, the formation of N-chloramine with NCS in polar protic solvents is accompanied by the formation of several by-products that significantly lower the yields and considerably complicate the final purification of N-chloramines.
- Strict anhydrous conditions are generally required to prevent the hydrolysis of acid chlorides, ketenes and imines.
- As expected, the 1H-NMR spectrum of the crude reaction product showed the presence of a mixture of cis and trans isomers in a 92
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 ratio. These data fit those described in literature for the classic Staudinger reaction, where a cis selectivity is typically observed using acid chlorides activated with heteroatoms at the alpha position.
8 ratio. These data fit those described in literature for the classic Staudinger reaction, where a cis selectivity is typically observed using acid chlorides activated with heteroatoms at the alpha position. - These results were not entirely unexpected, as the preparation of N-chloroanilines is extremely complex and N-chloroanilines are highly unstable compounds.
- To our knowledge, there are relatively few literature precedents concerning the use of non-activated aliphatic acid chlorides in the synthesis of β-lactams M. Browne, D. A. Burnett, M. A. Caplen, L.-Y. Chen, J. W. Clader, M. Domalski, S. Dugar, P. Pushpavanam, R. Sher, W. Vaccaro, M. Viziano and H. Zhao, Tetrahedron Lett., 1995, 36, 2555–2558 CrossRef CAS; C. M. L. Delpiccolo and E. G. Mata, Tetrahedron Lett., 2004, 45, 4085–4088 CrossRef.
- Several unsuccessful attempts were conducted using NEt3, tBuOK, DBA–DBU, pyridine and KO2 as base.
- SciFinder database.
- G. I. Georg, Organic Chemistry of β-Lactams, Wiley VCH, New York, 1992 CrossRef CAS PubMed; F. P. Cossio, A. Arrieta and M. A. Sierra, Acc. Chem. Res., 2008, 41, 925–936 CrossRef CAS PubMed; Y. Liang, L. Jiao, S. Zhang, Z.-X. Yu and J. Xu, J. Am. Chem. Soc., 2009, 131, 1542–1549 CrossRef PubMed; H. Qi, X. Lia and J. Xu, Org. Biomol. Chem., 2011, 9, 2702–2714 Search PubMed; J. Xu, ARKIVOC, 2009, 21–44 Search PubMed; S.-L. Hou, X.-Y. Li and J. X. Xu, Sci. China: Chem., 2013, 56, 370–379 CrossRef; X. Z. Yang, N. Chen and J. Xu, J. Org. Chem., 2015, 80, 3611–3620 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra02744j |
| This journal is © The Royal Society of Chemistry 2016 |