Carbon quantum dots/Ni–Al layered double hydroxide composite for high-performance supercapacitors†
Abstract
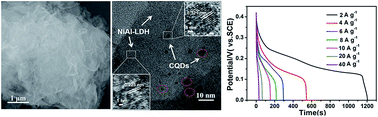
Ultrathin nanoplate-like carbon quantum dots (CQDs)/Ni–Al layered double hydroxide (LDH) composite has been fabricated by a facile one-step solvothermal method. The obtained porous CQDs/NiAl-LDH composite displayed an enlarged specific surface and nanoscale size. As supercapacitor electrode material, the composite exhibited remarkable electrochemical performance with superior specific capacitance of 1794 F g−1 (at 2 A g−1), high rate capability of 967 F g−1 (at 20 A g−1) and excellent cycle performance (about 93% retention over 1500 cycles). The enhanced supercapacitor performance of CQDs/NiAl-LDH electrode should be attributed to the synergistic effect between NiAl-LDH nanosheets and CQDs.


 Please wait while we load your content...
Please wait while we load your content...