Interfacial self-assembly of nanoporous C60 thin films†
Jean-Nicolas Tisserant*a,
Patrick A. Reissnera,
Sandra Jenatschb,
Hannes Beyera,
Roland Hanyb and
Andreas Stemmer*a
aETH Zürich, Nanotechnology Group, Säumerstrasse 4, CH-8803 Rüschlikon, Switzerland. E-mail: jeanti@ethz.ch; astemmer@ethz.ch
bEmpa, Swiss Federal Laboratories for Materials Science and Technology, Laboratory for Functional Polymers, Überlandstrasse 129, CH-8600 Dübendorf, Switzerland
First published on 22nd February 2016
Abstract
Nanoporous surface morphologies often perform better than their planar or bulk counterparts in phenomena such as catalysis and charge transport. In organic photovoltaics, for example, fullerene films structured on a scale corresponding to the exciton diffusion length, typically a few tens of nanometers, could improve charge separation. Existing methods to form such films, however, typically do not reach features smaller than 100 nm. Here, we propose a simple method based on interfacial nucleation and growth to produce large and flexible 2D percolating assemblies of C60 nanoparticles with diameters below 50 nm that cover up to 60% of the surface. These films can be rendered insoluble by photopolymerization and infiltrated with other functional molecules. We illustrate the benefit of such stabilized nanoporous films on the example of organic solar cells of the architecture ITO/TiO2/C60/P3HT/MoO3/Ag. Structured C60 films with interconnected morphological features perform better than planar C60 films by 50% on average, reaching a power conversion efficiency of 1.3%. Due to the ease of film fabrication and small dimensions of the C60 morphological features, these results may find application beyond organic photovoltaics, in a variety of fields where an enhanced interface with a fullerene surface is needed.
Introduction
The properties of nanomaterials in general, and porous materials in particular, differ from those of their bulk counterparts because surface and interface phenomena play an important role on the nanometer scale.1 Enhanced interfaces compared to planar counterparts increase the efficiency of nanoporous materials in processes like catalysis,2 gas storage,3 molecular recognition,4 separation,5 and solar energy conversion.6,7Carbon-rich materials such as fullerenes have played a fundamental role in the development of modern nanoscience and technology.8,9 Nanoporous C60 films are for example used in catalytic methanol oxidation10 or as electrode materials in aqueous solutions.11 Beyond these two examples, the outstanding opto-electronic properties of C60 associated with a versatile chemistry makes fullerenes interesting for optics12 or biology13 and essential for the entire organic photovoltaic (OPV) field where fullerenes are widely used as electron acceptor materials.14–18 For such an application, nanoporous C60 thin films would be highly desirable because excitons are split into free charge carriers at molecular heterojunctions.7 In many cases, active materials have been deposited as bilayers,14,15,19,20 or interdigitated heterojunctions, usually using soluble fullerene derivatives21–23 rather than pristine C60.24 Nanoporous C60 films with morphological features in the range of the typical exciton diffusion length, ∼10–20 nm in comparable systems,16,25,26 would offer an attractive alternative to bilayers in bi-component systems that do not phase-separate spontaneously to form an optimal bulk heterojunction morphology.
The fabrication of porous two-dimensional films of fullerenes on a large scale remains challenging. Some attempts were made by sacrificial templating,27 electrodeposition from colloids,28,29 or self-assembly of synthesized C60 nanoparticles.30 These methods remain limited by the difficulty to prepare C60 nanoparticles smaller than 100 nm in diameter.30–32 Interfacial self-assembly has emerged as a promising method for the fabrication of free-floating films that can be readily integrated into functional devices.33,34 In particular, progress in the field of metallic nanoparticle assembly35 inspired the interfacial technique that we present here to form single-layer or multilayer two-dimensional C60 films having morphological features below 50 nm in diameter. Rapid solvent evaporation on an air/water interface is responsible for the formation of 2D interdigitated networks, typical of diffusion-limited colloid aggregation (DLCA).36 The increase of interface area resulting from these nanostructures exceeds 30%. Importantly, the films are floating on a liquid surface and are flexible, allowing further transfer onto any substrate.
Pristine C60 is known to photo-polymerize in the solid state in a 2 + 2 cycloaddition process.37–40 This polymerization has for example been used to pattern fullerene films,41 but was also identified as a degradation pathway that limits long term OPV cell performance.40,42 Here, we use C60 photopolymerization to stabilize optimal fullerene morphologies against further solvent processing, similarly to results obtained by chemically modifying the fullerene.26,43–45 Nanoporous films were stabilized, infiltrated with a well-known electron-donor material, poly(3-hexylthiophene-2,5-diyl) (P3HT) – resulting in finely inter-penetrating C60/P3HT domains – and implemented as photoactive layer in C60/P3HT solar cells. By adding a nanoporous C60 layer to the bilayer configuration, the enhanced interface and the film morphology adapted to the exciton diffusion length resulted in an improvement of 50% on average and up to 80% in the power conversion efficiency (PCE), reaching η = 1.3%.
Experimental
Formation of C60 films
5 mg of C60 (Aldrich, 99.9%) were ground to fine powder in a 5 mL glass vial. 5 mL of chloroform (Sigma Aldrich, >99.8%) were added, the mixture was sonicated 30 minutes and left overnight to settle. C60 films were formed at the interface between water and air, in a 10 mL round poly(tetrafluoroethylene) (PTFE) beaker with a diameter of 2.1 cm by pouring dropwise 5 to 500 μL of saturated C60 solution on the convex water meniscus formed upon contact with PTFE. The films formed a thin brown and immobile layer on top of the water meniscus within a few minutes. Optionally, the C60 solution was deposited under reduced pressure. If not stated otherwise, 50 μL of solution were deposited at a pressure of 500 mbar. To upscale the film area to several cm2 for solar cell fabrication on 2.5 × 2.5 cm2 large substrates, 100 μL of saturated C60 solution were deposited drop wise in a 3.8 cm wide PTFE beaker without applying vacuum.Transfer to substrate
The films were transferred to Si/SiO2 or glass by dipping the substrate vertically in the beaker and retracting it through the floating film. No double layers were formed by this process. To form multilayers, the same substrate was dipped and retracted through multiple freshly assembled films.Stabilization and infiltration
C60 films were stabilized by light exposure (1 sun) overnight under inert atmosphere, at least 6 h are necessary according to previously published results.40 The solubility of stabilized films was tested by spin-coating (1000 rpm, 60 s) pure chloroform (1 mL on 4 cm2). Regioregular P3HT (Rieke Speciality Polymers) was dissolved in chloroform (1.25 to 10 mg mL−1) and spin-coated (1000 rpm, 30 s) on stabilized C60 films.Solar cell fabrication
Except for nanoporous C60 film deposition, all device fabrication steps were done in a glove box under nitrogen (H2O < 1 ppm, O2 < 10 ppm). ITO substrates (Geomatec, sheet resistance 20 ohm square−1) were cleaned and coated with TiO2 (50 nm).46 To this end, 0.95 g of Ti-(O-iC3H7)4 (Aldrich, 99.9%) were diluted in 10 mL of ethanol and stirred at 0 °C. A solution consisting of concentrated HCl (Sigma-Aldrich 0.03 g), water (0.135 g) and ethanol (10 mL) was added while stirring. The mixture was stirred 30 min at 0 °C before spin-coating (step 1: 5 s, 300 rpm s−1 to 1000 rpm; step 2: 60 s, 3000 rpm s−1, 4000 rpm). The films were annealed to 460 °C in 3 h, kept at 460 °C for 2 h, and cooled to room temperature. TiO2 films were heated to 120 °C on a hot plate under nitrogen (10 min) before depositing any further layer. C60 nanostructured films were deposited on TiO2 directly or on a 30 nm thick evaporated C60 layer. The films were dried on a hotplate (80 °C) for 6 h under nitrogen to remove water and exposed to 1 sun illumination overnight under nitrogen to photo-polymerize C60.40 Planar C60 films were deposited by thermal evaporation at <5 × 10−6 mbar. The active layer was covered with 10 nm of thermally evaporated MoO3 (Sigma Aldrich, 99.99%) as a buffer layer and 70 nm of Ag (Cerac, 99.99%) as an electrode. The active area of the cells was 0.031 or 0.071 cm2.Characterization
Scanning electron microscopy (SEM) was performed on a Hitachi SU8000 at a typical voltage of 1.5 kV. Images were analyzed using ImageJ. To evaluate lateral dimensions of the assemblies, SEM images of 1.2 × 1.2 μm2 were binarized using an Otsu threshold. The line plot of such an image shows maxima at the averaged position of interfaces. Scanning force microscopy (SFM) was performed on an MFP-3D (Asylum Research) with Olympus AC160TS-R3 cantilevers. TEM was performed on a Philips CM30 operated at a voltage of 300 kV. Devices were transferred into an air-tight transfer box for J–V characteristics measurement under a solar simulator delivering a light intensity of 100 mW cm−2. Photoluminescence was measured on a Fluorolog (Horiba Jobin-Yvon).Results & discussion
Nanoporous C60 thin films
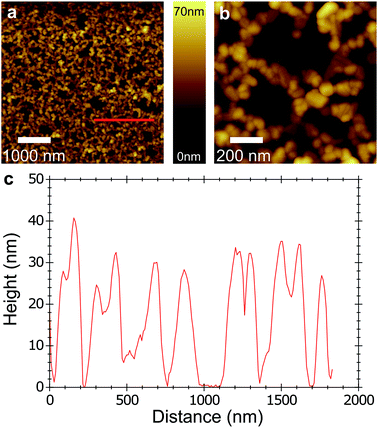
Two-dimensional nanostructured thin films of C60 were formed by evaporation of C60-saturated chloroform solutions at the convex interface formed between water and air in a PTFE beaker (Fig. 1a). The films obtained are percolating networks of C60 particles forming a single layer (Fig. 1b), covering surfaces up to several square centimeters. A 400 μm2 area is shown in Fig. S1, ESI.† The films are floating on the water meniscus, allowing their transfer onto diverse substrates including glass, silicon, or metals. An example of a single layer 2D film deposited on silicon is shown in Fig. 1b and c. Multilayer nanoporous films could be produced by successive deposition of films on the same surface (Fig. 1d). The nanoporous films exhibit astonishing mechanical flexibility and can, for example, effectively cover pronounced geometrical kinks (Fig. S2, ESI†). This flexibility can be explained by the film morphology. SFM revealed that the C60 film is composed of agglomerated nanoparticles (Fig. 2a and b). The typical height of the C60 particles is 20–30 nm (Fig. 2c). The total C60 surface coverage reached up to 60%. Transmission electron microscopy (TEM) showed that the films are indeed composites of crystalline C60 nanoparticles between 10 and 50 nm bound together by amorphous C60 (Fig. S3, ESI†). The film shown in Fig. 2 has a 36% increased surface area compared to a plain C60 film, considering the C60 particles as spheres.A similar convex interface was already used to self-assemble 2D films of metallic nanoparticles.35 Dushkin et al. measured and modeled the self-assembly of latex particles on water meniscus.47 They found that latex colloidal crystals nucleate at the center of the meniscus and grow radially under capillary action until they cover most of the surface. This comparison, along with our morphological observations allows us to interpret the C60 film formation as follows: as the chloroform solution is suddenly brought into supersaturation under solvent evaporation, multiple nucleation events occur at the air/solvent interface. The mobile C60 clusters thus formed may in parallel either grow radially or aggregate on other clusters, forming morphologies typical of diffusion-limited colloid aggregation with multiple seeds.36 This model was tested by varying the amount of solution deposited on the water meniscus and the surrounding pressure.
Tuneable film properties
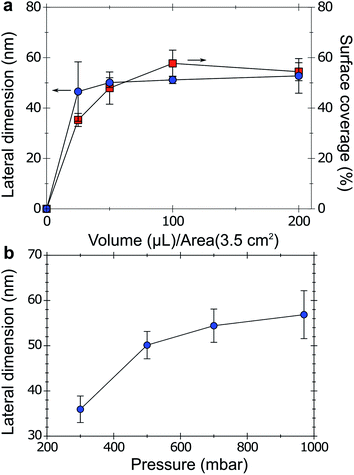
Varying the volume of C60 solution deposited on the water meniscus allows tuning the total surface coverage with little variations of the lateral dimensions of the C60 structures (Fig. 3a). The surface coverage could be varied between 37% and 60% by depositing 25 to 200 μL of C60-saturated chloroform solution on 3.5 cm2 of interface (Fig. 3a). Below 25 μL, the film only partially covered the water meniscus. Above 250 μL, large C60 crystals formed at the air/water interface, leading to film inhomogeneity and no increased coverage (Fig. S4, ESI†). In order to probe the effect of solvent evaporation time, 50 μL of saturated chloroform were deposited under pressures varying from 300 to 1000 mbar (Fig. 3b). The surface coverage did not vary during this experiment. Lowering the surrounding pressure leads to the formation of smaller lateral dimensions of the assemblies, reaching 35 nm at 300 mbar. The lower pressure limit is given by the boiling point of the carrier liquid water. In summary, C60 coverage is mainly controlled by the volume of deposited solution, i.e. the number of nucleation events per unit surface, and the size of the resulting features is mainly controlled by the particle growth time, when solution and stable nuclei coexist. These two parameters, evaporation time and volume of deposited C60 solution allow tuning the morphological outcome of C60 thin films self-assembled on an air/water interface. The method was scaled up for film sizes over 11 cm2 (see Experimental section). Films obtained on such a large scale showed particles that were smaller than 25 nm with a surface coverage above 50% (Fig. 4).Morphology stabilization and P3HT infiltration
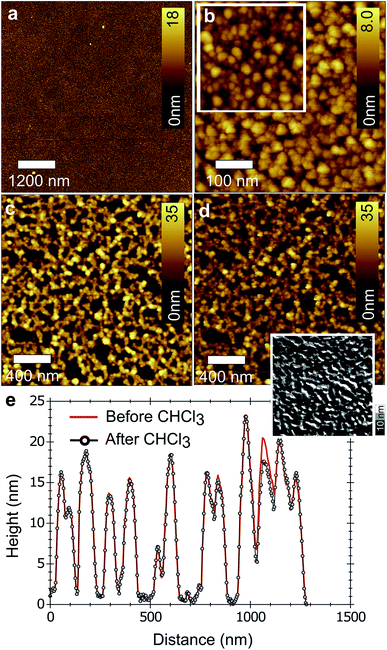
For the versatile use of nanostructured C60 films, the ability to freeze their optimal morphology against further processing or ageing is appealing.26,43–45 It is known that C60 photo-polymerizes in the solid state, yielding poorly soluble species.40 This reaction is used here to stabilize nanoporous C60 films against solvent processing. Solvent rinsing was carried out in a way that mimics the conditions under which films were treated during solar cell processing. The insolubility of flat polymerized C60 films reached almost 100% (Fig. 4a and b). For nano-structured C60 films, SFM measurements (Fig. 4c–e) of the same area before and after solvent rinsing showed an overall insolubility of 84 ± 15% (average of 5 samples). A reference SEM image demonstrating the pronounced solubility of a C60 film that was not polymerized is shown in Fig. S5, ESI.† We found a similar level of stability against solvent rinsing for as-prepared films, and for films used for solar cell fabrication that were annealed and dried before photo-stabilization.The insolubility of stabilized nanoporous C60 films allows infiltrating them with a second material from the same casting solvent. P3HT coated from chloroform was taken as an example to illustrate the concept (see Fig. S6, ESI†). The concentration of P3HT was optimized to completely cover the C60 nanostructures. The peak-to-valley amplitude was reduced from 72 nm to 3.3 nm by increasing the P3HT concentration up to 10 mg mL−1. Films coated at 5 mg mL−1 resulted in an optimal morphology and were chosen for the rest of the study. High-resolution SFM (see Fig. S7, ESI†) and SEM (Fig. 5) were used to prove that P3HT chains effectively infiltrate the nanoscopic voids in C60 films.
 | ||
| Fig. 5 Use of nanoporous C60 films (S-C60) in solar cells. (a) SEM cross-section image of the S-C60 cell without top contact; (b) SEM cross section of the S-C60/F-C60 cell. | ||
Use in solar cells
The merits of a C60 small-scale stabilized particle network can be demonstrated via improved charge separation in organic photovoltaics.7 Devices with the configuration ITO/TiO2/C60/P3HT/MoO3/Ag were fabricated to measure the effect of C60 nano-morphology on cell performance. The C60 layer was either flat (40 nm evaporated C60, named F-C60), or nanostructured as described above (S-C60) or a stack of both structures, S-C60 on 30 nm F-C60. 30 nm F-C60 films were chosen in this case to keep the film absorption constant. Removing water from S-C60 films before illumination was done by thermally annealing the samples under nitrogen (6 h, 80 °C). Drying induced a slight coarsening of the morphology. The change in morphology and the insolubility of films that were dried on a hotplate before polymerization are shown in Fig. S8, ESI.† Solar cell performance data are summarized in Table 1. First, C60 stabilization was essential to device performance as pristine C60 is largely dissolved when attempting to infiltrate P3HT from a chloroform solution. Consequently, the performance of non-stabilized F-C60/P3HT cells was poor (Table 1, entry a). Stabilized F-C60/P3HT cells reached on average η = 0.67% with JSC = 2.5 mA cm−2, VOC = 0.48 V, FF = 56.8% (Table 1, entry b). S-C60 alone, even when stabilized, performed less than F-C60 (Table 1, entry c and d). This can be ascribed to the lower electron acceptor content in a S-C60 film, as compared to F-C60, and P3HT contacting directly the TiO2 layer. These two adverse effects were reduced by stacking two S-C60 layers on top of each other (Table 1, entry e), and suppressed by depositing S-C60 on top of F-C60 (Table 1, entry f). In the latter case, and for a constant total cell absorption, the average cell performance was increased by ∼50% compared to the flat film. Adding a further S-C60 layer increased the best device performance further (Table 1, entry g).| Entry | Structure of C60 layer | VOC [V] | JSC [mA cm−2] | FF [%] | η [%] | ηmax [%] |
|---|---|---|---|---|---|---|
| a F-C60 denotes a planar C60 film, S-C60 a nanostructured film. Solar cell performance parameters are the average and standard deviation (s.d) values of a batch of more than 11 identically processed devices. VOC denotes the open-circuit voltage, JSC the short-circuit current, FF the fill factor, and η the power conversion efficiency. | ||||||
| a | F-C60 no light | 0.29 (0.10) | 1.3 (0.4) | 29.6 (9.5) | 0.13 (0.1) | 0.27 |
| b | F-C60 light | 0.48 (0.02) | 2.5 (0.2) | 56.8 (2.3) | 0.67 (0.1) | 0.71 |
| c | 1 layer S-C60 no light | 0.16 (0.04) | 0.43 (0.1) | 30.8 (3.0) | 0.02 (0.01) | 0.03 |
| d | 1 layer S-C60 light | 0.42 (0.08) | 0.92 (0.3) | 45.9 (5.8) | 0.18 (0.1) | 0.27 |
| e | 2 layers S-C60 light | 0.48 (0.02) | 1.50 (0.7) | 46.4 (2.2) | 0.32 (0.1) | 0.41 |
| f | 1 layer S-C60 on F-C60 light | 0.52 (0.004) | 3.3 (0.3) | 59.0 (1.6) | 1.0 (0.1) | 1.14 |
| g | 2 layers S-C60 on F-C60 light | 0.47 (0.09) | 3.4 (0.9) | 53.2 (5.4) | 0.87 (0.3) | 1.28 |
Cross-section SEM images of S-C60 and S-C60 on F-C60 active layers are shown in Fig. 5a and b, respectively, and a cross-section SEM image of F-C60 active layer is shown in Fig. S9, ESI.† The SEM images reveal an enhanced interface after adding S-C60 on F-C60. From SFM data shown earlier, an increase of the interface area of ∼18% can be calculated by considering each C60 feature as half-spherical, and the interface increase is about 36% for spherical structures. From this, it is clear that the 50% average increase in the cell efficiency and in particular the 32% increase in JSC upon adding one S-C60 layer onto F-C60 is mainly due to an increase of the C60/P3HT interface. This hypothesis is also supported by photoluminescence experiments. The difference between P3HT emission on F-C60 and on S-C60/F-C60 is close to 30% (Fig. S10, ESI†). Internal photon-to-current conversion efficiency (IPCE) data from F-C60 and 2 × S-C60 on F-C60 devices are shown in Fig. S11, ESI.† The IPCE maximum value was 30% for F-C60 and 42% for 2 × S-C60 on F-C60.
Reports on C60/P3HT solar cells are scarce, because of the low solubility of C60 in organic solvents and because the two components do not spontaneously phase-separate to form an optimal bulk-heterojunction morphology, limiting drastically the cell performance.24 One solution found by Geiser et al. was to anneal the whole device to force the morphology to evolve favorably, reaching over 2% in PCE.24 Even if the final PCE shown here does not reach that of bulk-heterojunction fullerene/P3HT devices, the solution proposed, using a C60 film stabilized in an optimal morphology, can be seen as rather universal and opens important improvement possibilities for other working systems.
Conclusions
In this article we have shown a new method based on interfacial nucleation-and-growth to form nanoporous C60 films. They can be described as 2D percolating networks of C60 nanoparticles with diameters below 50 nm and as small as 10 nm. The particles are the smallest and most monodisperse reported to date. Both single-layer and multilayer films could be readily fabricated, stabilized against further solvent processing, and the process was up-scaled to several square centimeters. The lateral dimensions of C60 domains below 50 nm make such thin film morphologies highly adapted for any physical or chemical process where an enhanced interface is advantageous. We chose to illustrate this by the example of increased performance in organic P3HT-based solar cells, accomplished by an enhanced C60/P3HT interface and adapted donor/acceptor morphology. However, we believe that our assembly method may be extended beyond fullerenes and OPV, to other functional molecules and fields where enhanced interface matters, such as catalysis, sensing or fuel cells.Acknowledgements
The authors thank the staff of the BRNC, Rüschlikon for support, J. E. Quinsaat (Empa Dübendorf) for TEM imaging, M. Makha (Empa Dübendorf) for providing TiO2 substrates, C. Ruiz-Vargas, N. Mojarad (ETH) for helpful comments, and F. Nüesch (Empa Dübendorf) for support.References
- A. Zangwill, Physics at Surfaces, Cambridge University Press, Cambridge, UK, 1988 Search PubMed.
- H. L. Ngo and W. Lin, Top. Catal., 2005, 34, 85–92 CrossRef CAS.
- H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 8875–8883 CrossRef CAS PubMed.
- D. Bonifazi, S. Mohnani and A. Llanes-Pallas, Chemistry, 2009, 15, 7004–7025 CrossRef CAS PubMed.
- Q. Min Wang, D. Shen, M. Bülow, M. Ling Lau, S. Deng, F. R. Fitch, N. O. Lemcoff and J. Semanscin, Microporous Mesoporous Mater., 2002, 55, 217–230 CrossRef.
- J. Burschka, N. Pellet, S.-J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Grätzel, Nature, 2013, 499, 316–319 CrossRef CAS PubMed.
- S. E. Gledhill, B. Scott and B. A. Gregg, J. Mater. Res., 2011, 20, 3167–3179 CrossRef.
- H. W. Kroto, A. W. Allaf and S. P. Balm, Chem. Rev., 1991, 91, 1213–1235 CrossRef CAS.
- J. M. Ball, H. W. Paul and T. D. Anthopoulos, in Advances in Carbon Nanomaterials: Science and Applications, ed. N. Tagmatarchis, Pan Stanford Publishing Pte. Ltd., 2012, vol. 78, pp. 978–981 Search PubMed.
- K. Vinodgopal, M. Haria, D. Meisel and P. Kamat, Nano Lett., 2004, 4, 415–418 CrossRef CAS.
- A. Szucs, A. Loix, J. B. Nagy and L. Lamberts, Synth. Met., 1996, 77, 227–230 CrossRef CAS.
- X. L. Jenekhe and S. A. Chen, Science, 1999, 283, 372–375 CrossRef PubMed.
- A. W. Jensen, S. R. Wilson and D. I. Schuster, Bioorg. Med. Chem., 1996, 4, 767–779 CrossRef CAS PubMed.
- N. S. Sariciftci, D. Braun, C. Zhang, V. I. Srdanov, A. J. Heeger, G. Stucky and F. Wudl, Appl. Phys. Lett., 1993, 62, 585 CrossRef CAS.
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789–1791 CrossRef CAS.
- P. Peumans, A. Yakimov and S. R. Forrest, J. Appl. Phys., 2003, 93, 3693 CrossRef CAS.
- A. W. Hains, Z. Liang, M. A. Woodhouse and B. A. Gregg, Chem. Rev., 2010, 110, 6689–6735 CrossRef CAS PubMed.
- H. Spanggaard and F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2004, 83, 125–146 CrossRef CAS.
- C. W. Tang, Appl. Phys. Lett., 1986, 48, 183 CrossRef CAS.
- J. J. M. Halls, K. Pichler, R. H. Friend, S. C. Moratti and A. B. Holmes, Appl. Phys. Lett., 1996, 68, 3120 CrossRef CAS.
- F. Yang, M. Shtein and S. R. Forrest, Nat. Mater., 2004, 4, 37–41 CrossRef.
- M. Granström, K. Petritsch, A. C. Arias, A. Lux, M. R. Andersson and R. H. Friend, Nature, 1998, 395, 257–260 CrossRef.
- J. Xue, B. P. Rand, S. Uchida and S. R. Forrest, Adv. Mater., 2005, 17, 66–71 CrossRef CAS.
- A. Geiser, B. Fan, H. Benmansour, F. Castro, J. Heier, B. Keller, K. E. Mayerhofer, F. Nüesch and R. Hany, Sol. Energy Mater. Sol. Cells, 2008, 92, 464–473 CrossRef CAS.
- H. Hoppe and N. S. Sariciftci, J. Mater. Chem., 2006, 16, 45–61 RSC.
- D. E. Markov, E. Amsterdam, P. W. M. Blom, A. B. Sieval and J. C. Hummelen, J. Phys. Chem. A, 2005, 109, 5266–5274 CrossRef CAS PubMed.
- J. Heier, R. Steiger, F. Nüesch and R. Hany, Langmuir, 2010, 26, 3955–3961 CrossRef CAS PubMed.
- S. Barazzouk, S. Hotchandani and P. V. Kamat, Adv. Mater., 2001, 13, 1614–1617 CrossRef CAS.
- H.-G. Jeon, S. Ryo, T. Sugiyama, I. Oh, H. Masuhara and T. Asahi, Chem. Lett., 2007, 36, 1160–1161 CrossRef CAS.
- R. Kudo, J. Matsui, T. Yokoyama, A. Masuhara, H. Kasai, H. Oikawa and T. Miyashita, Mol. Cryst. Liq. Cryst., 2011, 539, 408–412 Search PubMed.
- S. Deguchi, R. G. Alargova and K. Tsujii, Langmuir, 2001, 17, 6013–6017 CrossRef CAS.
- R. G. Alargova, S. Deguchi and K. Tsujii, J. Am. Chem. Soc., 2001, 123, 10460–10467 CrossRef CAS PubMed.
- X. Ye, J. E. Collins, Y. Kang, J. Chen, D. T. N. Chen, A. G. Yodh and C. B. Murray, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 22430–22435 CrossRef CAS PubMed.
- X. Ye, J. Chen, M. Engel, J. A. Millan, W. Li, L. Qi, G. Xing, J. E. Collins, C. R. Kagan, J. Li, S. C. Glotzer and C. B. Murray, Nat. Chem., 2013, 5, 466–473 CrossRef CAS PubMed.
- V. Santhanam, J. Liu, R. Agarwal and R. P. Andres, Langmuir, 2003, 19, 7881–7887 CrossRef CAS.
- M. Y. Lin, H. M. Lindsay, D. A. Weitz, R. C. Ball, R. Klein and P. Meakin, Nature, 1989, 339, 360–362 CrossRef CAS.
- P. C. Eklund, A. M. Rao, P. Zhou, Y. Wang and J. M. Holden, Thin Solid Films, 1995, 257, 185–203 CrossRef CAS.
- A. M. Rao, P. Zhou, K. A. Wang, G. T. Hager, J. M. Holden, Y. Wang, W. T. Lee, X. X. Bi, P. C. Eklund, D. S. Cornett, M. A. Duncan and I. J. Amster, Science, 1993, 259, 955–957 CrossRef CAS.
- M. Suzuki, T. Iida and K. Nasu, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 2188–2198 CrossRef CAS.
- H. Zhang, A. Borgschulte, F. A. Castro, R. Crockett, A. C. Gerecke, O. Deniz, J. Heier, S. Jenatsch, F. Nüesch, C. Sanchez-Sanchez, A. Zoladek-Lemanczyk and R. Hany, Adv. Energy Mater., 2015, 5, 1400734 CrossRef.
- J. Wang, C. Larsen, T. Wågberg and L. Edman, Adv. Funct. Mater., 2011, 21, 3723–3728 CrossRef CAS.
- G. Wicht, S. Bücheler, M. Dietrich, T. Jäger, F. Nüesch, T. Offermans, J.-N. Tisserant, L. Wang, H. Zhang and R. Hany, Sol. Energy Mater. Sol. Cells, 2013, 117, 585–591 CrossRef CAS.
- J.-F. Nierengarten and S. Setayesh, New J. Chem., 2006, 30, 313 RSC.
- J.-N. Tisserant, R. Hany, E. Wimmer, A. Sánchez-Ferrer, J. Adamcik, G. Wicht, F. Nüesch, D. Rentsch, A. Borgschulte, R. Mezzenga and J. Heier, Macromolecules, 2014, 47, 721–728 CrossRef CAS.
- M. Drees, H. Hoppe, C. Winder, H. Neugebauer, N. S. Sariciftci, W. Schwinger, F. Schäffler, C. Topf, M. C. Scharber, Z. Zhu and R. Gaudiana, J. Mater. Chem., 2005, 15, 5158 RSC.
- E. Berner, T. Jäger, T. Lanz, F. Nüesch, J.-N. Tisserant, G. Wicht, H. Zhang and R. Hany, Appl. Phys. Lett., 2013, 102, 183903 CrossRef.
- C. D. Dushkin, G. S. Lazarov, S. N. Kotsev, H. Yoshimura and K. Nagayama, Colloid Polym. Sci., 1999, 277, 914–930 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: SEM images of large area nanostructured C60 films, SEM images of S-C60 deposited on a 90° geometric kink, a TEM image of S-C60, an SEM image of large C60 crystals, SEM images showing the solubility of S-C60 films that were not illuminated, SFM images of P3HT infiltration, an SFM study of S-C60 films upon drying, an SEM side-image of a F-C60/P3HT solar cell, P3HT photoluminescence data, and IPCE measurements of characteristic solar cells. See DOI: 10.1039/c6ra02720b |
| This journal is © The Royal Society of Chemistry 2016 |