Antimicrobial and fluorescence finishing of woolen yarn with Terminalia arjuna natural dye as an ecofriendly substitute to synthetic antibacterial agents†
Abstract
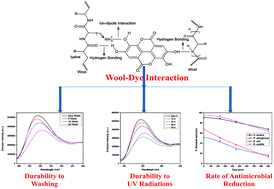
The current study deals with the use of Terminalia arjuna natural dye as an ecofriendly finishing agent for producing highly functional antimicrobial and fluorescent woolen yarn along with the evaluation of kinetic and thermodynamic adsorption characteristics. The effect of pH on the adsorption was investigated, showing an increase in adsorption capacity with decreasing pH over the range of 2–9, with maximum adsorption at pH 3.5. Two kinetic equations pseudo-first order and pseudo-second order were employed for determining adsorption rates. The pseudo-second order equation provided the best fit to experimental data with an activation energy of 105.58 kJ mol−1, indicating chemisorption. The equilibrium adsorption data was fitted to Langmuir, Freundlich and Redlich–Peterson adsorption isotherms. The adsorption behavior accorded with the Redlich–Peterson isotherm with exceptionally high regression coefficients for dyeing temperatures of 50, 70 and 90 °C with dye concentration varying from 0.5–10% (o.w.f). Comparative results of the colorimetric properties (CIEL*a*b* and K/S) using a spectrophotometer under D65 illuminant (10° standard observer) and color fastness (light, wash, and rub) of dyed woolen yarns were studied to quantify the effect of metal mordants. The antimicrobial potential of Terminalia arjuna solution and dyed woolen yarn were assessed in terms of percentage inhibition of bacterial growth against a wide variety of bacterial strains, showing more than 85% inhibition. Reduction in antimicrobial activity of dyed woolen yarn was observed with mordanted samples, however they were found to retain more antimicrobial activity as compared to unmordanted samples as a function of successive washing cycles. The chemical nature of different mordants and wool–mordant–dye complex forming ability were found to have significant impact on the colorimetric and fluorescence characteristics of dyed woolen yarn.

 Please wait while we load your content...
Please wait while we load your content...