Low-temperature sol–gel processed AlOx gate dielectric buffer layer for improved performance in pentacene-based OFETs†
Abstract
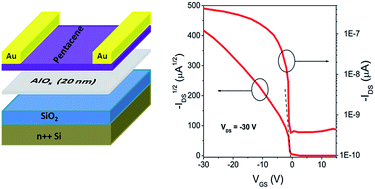
An approach to achieve improved performance in pentacene-based organic field effect transistors (OFETs) using high-k AlOx prepared by a low temperature sol–gel technique as a thin buffer layer on a SiO2 gate dielectric was demonstrated. The maximum processing temperature for the AlOx thin layer was 150 °C. The resulting all-inorganic SiO2/AlOx bilayer gate dielectric system exhibited a low leakage current density <1 × 10−8 A cm−2 under an applied electric field strength of 1.8 MV cm−1, a smooth surface with an rms of 0.11 nm and an equivalent dielectric constant (k) of 4.13. The OFET fabricated as a result of this surface modification exhibited a significantly improved field effect mobility of 0.81 cm2 V−1 s−1 when compared with a reference device with a SiO2 single layer gate dielectric, which had a lower mobility of 0.28 cm2 V−1 s−1.

 Please wait while we load your content...
Please wait while we load your content...