Hierarchical LiNi0.8Co0.15Al0.05O2 plates with exposed {010} active planes as a high performance cathode material for Li-ion batteries†
Zhen
Wang
ab,
Heng
Liu
a,
Jian
Wu
b,
Woon-Ming
Lau
b,
Jun
Mei
b,
Hao
Liu
*b and
Guobiao
Liu
*b
aCollege of Materials Science and Engineering, Sichuan University, Chengdu 610065, China
bChengdu Green Energy and Green Manufacturing Technology R&D Center, Chengdu Development Center of Science and Technology, China Academy of Engineering Physics, Chengdu 610207, China. E-mail: mliuhao@gmail.com; guobiaoliu@sina.com
First published on 22nd March 2016
Abstract
Exposed {010} active planes can provide more tunnels for Li+ insertion/extraction through the particle surface. In this work, hierarchical LiNi0.8Co0.15Al0.05O2 plates with exposed {010} active planes were successfully obtained via a solvothermal method followed by solid-state reaction. The as-synthesized hierarchical LiNi0.8Co0.15Al0.05O2 plates demonstrate a superior rate capability.
Introduction
Because of its high capacity, low toxicity and low cost, over the past twenty years, LiNiO2 has been considered as an alternative cathode material to commercialized LiCoO2.1–5 Nevertheless, pristine LiNiO2 demonstrates poor thermodynamic stability, a property which hinders its commercialization. Through various research efforts, it was found that Co and Al co-doping can greatly improve its thermodynamic stability and electrochemical reactivity.6–8 So far, Co and Al co-doped LiNi0.8Co0.15Al0.05O2 has been successfully put into practical use for lithium ion batteries (LIBs) used in electric vehicles (EVs). However, to satisfy the demand for higher rate capabilities, higher energy densities and longer life for advanced LIBs so that EVs can run faster and travel longer distances, further improving the electrochemical performances of LiNi0.8Co0.15Al0.05O2 still attracts extensive attentions.To date, many efforts such as cation doping, surface modifying and morphological designing have been devoted to further improving the electrochemical performances of LiNi0.8Co0.15Al0.05O2.9–12 Among them, morphological designing of LiNi0.8Co0.15Al0.05O2 particles has been highlighted. For example, yolk–shell LiNi0.8Co0.15Al0.05O2 microspheres with a high discharge capacity and a good cyclic performance were obtained by an ultrasonic atomization method.13 Additionally, one-dimensional LiNi0.8Co0.15Al0.05O2 microrods with a fully improved electrochemical performance were synthesized via a two-steps co-precipitation method.14
However, as lithium ions diffusion in the α-NaFeO2 layered structure of LiNi0.8Co0.15Al0.05O2 occurs along the a (or b) axis through tunnels perpendicular to the {010} facets including (010), (0![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0), (100), (110), (1
0), (100), (110), (1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) and (
0) and (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 00) planes,15,16 designing LiNi0.8Co0.15Al0.05O2 plates with exposed {010} active planes is expected to be greatly helpful to further improve the electrochemical performances of LiNi0.8Co0.15Al0.05O2. While LiNi1/3Co1/3Mn1/3O2 and Li1.2Ni0.2Mn0.6O2 plates with {010} active planes have been achieved in previous literatures,15,17,18 research in this area has not been attempted on LiNi0.8Co0.15Al0.05O2 because of the difficulty in synthesis.17,18 Therefore, because of the high capacity, low toxicity and low cost of LiNi0.8Co0.15Al0.05O2, efforts to synthesis LiNi0.8Co0.15Al0.05O2 plates with exposed {010} active planes are well worth trying.
00) planes,15,16 designing LiNi0.8Co0.15Al0.05O2 plates with exposed {010} active planes is expected to be greatly helpful to further improve the electrochemical performances of LiNi0.8Co0.15Al0.05O2. While LiNi1/3Co1/3Mn1/3O2 and Li1.2Ni0.2Mn0.6O2 plates with {010} active planes have been achieved in previous literatures,15,17,18 research in this area has not been attempted on LiNi0.8Co0.15Al0.05O2 because of the difficulty in synthesis.17,18 Therefore, because of the high capacity, low toxicity and low cost of LiNi0.8Co0.15Al0.05O2, efforts to synthesis LiNi0.8Co0.15Al0.05O2 plates with exposed {010} active planes are well worth trying.
Herein, we demonstrate that LiNi0.8Co0.15Al0.05O2 plates with exposed {010} active planes can be prepared by using micro-sized flower-like particles as precursor which were obtained via a solvothermal method.
Experimental
The hierarchical LiNi0.8Co0.15Al0.05O2 plates with exposed {010} active planes were prepared using the micro-sized flower-like particles as precursor which were synthesized by a solvothermal method with PVP as the template and surfactant. These hierarchical plates were designated as HP-NCA. In order to make a comparison, we also prepared LiNi0.8Co0.15Al0.05O2 spherical micro-particles using commercial precursor. These spherical micro-particles were denoted as SP-NCA. The detailed Experimental section is shown in the ESI.†Results and discussion
The morphology of precursor plays a significant role on the preparation of plates with exposed {010} active planes. The morphology of the as-synthesized precursor is illustrated in Fig. 1a. As the figure shown, the as-synthesized flower-like precursor presents uniform and discrete micro-sized secondary spherical particles consisting of loosely connected plates with diameter above 500 nm. As a contrast, commercial precursor demonstrates the secondary spherical particles consisting of relatively compact connected plates with diameter below 300 nm (Fig. S1a, ESI†). The heated flower-like precursor shows a consistency with the as-synthesized one in Fig. 1b, except that the heated flower-like precursor demonstrates a great amount of nano-sized pores which should be caused by the gas release during the heating process. The neighboring plates of the heated flower-like precursor are also loosely connected, which would offer space for the plates to grow in the following calcining process. As a contrast, the heated commercial precursor shows almost the same morphology as the unheated one (Fig. S1b, ESI†). Fig. 1c and d display the morphologies of HP-NCA and SP-NCA. As shown in Fig. 1c, the morphology of HP-NCA basically inherits that of the heated precursor while the thickness of plates increases dramatically. The consistence between the morphology of HP-NCA and that of the heated precursor is in accordance with the previous result that the heated precursor can act as a stable self-template during the calcining process.18 By comparison, as shown in Fig. 1d, SP-NCA exhibits spherical secondary particles made up of compact and irregular shaped primary particles, an observation which is identical to the result in previous literatures.19–21 The morphology change for the primary particles of SP-NCA can be attributed to the strengthening of Ostwald ripening due to the relatively compact connected plates with smaller diameter.22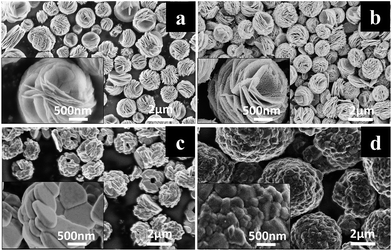 | ||
| Fig. 1 SEM images of (a) as-synthesized flower-like precursor, (b) heated flower-like precursor, (c) HP-NCA, (d) SP-NCA. Insets are the SEM images at high magnification. | ||
ICP analysis was taken to confirm the chemical composition by measuring the atomic ratios of Li, Ni, Co and Al in HP-NCA and SP-NCA. The calculated atomic ratios are shown in Table S1 (ESI†) and are approximately in accordance with those of LiNi0.8Co0.15Al0.05O2, a result which implies the homogeneous reaction during the lithiated process at high temperature.18 EDX-mapping images of Ni, Co and Al elements further confirm the homogeneous distribution of these elements in HP-NCA (Fig. S2, ESI†).
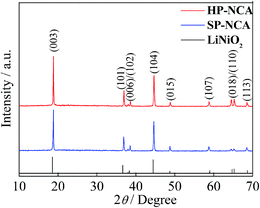
XRD patterns of HP-NCA and SP-NCA are shown in Fig. 2. The XRD patterns can be indexed as a typical α-NaFeO2 hexagonal structure with a space group of R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. All the diffraction peaks of HP-NCA and SP-NCA are consistent with those of LiNiO2 (JCPDS no. 74-0919) with exception of a slight shift of peak position toward higher degree. The introduction of Co3+ and Al3+ which have smaller radiuses (0.545 Å and 0.535 Å) in replacement of Ni3+ with a greater radius (0.600 Å) is believed to be responsible for the slight shift of peak position.23,24 The clear separation of (006)/(102) and (018)/(110) peak pairs for HP-NCA and SP-NCA suggests the formation of highly ordered hexagonal layered structure. Furthermore, the intensity ratios of (003) and (104) peaks are bigger than 1.2, which indicates a low extent of cation mixing.25 Notably, the intensity ratio of (003)/(104) for HP-NCA is 1.46 while that for SP-NCA is 1.31, a result which indicates that HP-NCA has a lower cation mixing than SP-NCA. This result can be attributed to the fact that the porous microstructure of the heated precursor for HP-NCA is in favor of improving reaction activity among Al2O3, Li2O and the heated precursor during the calcining process.
m. All the diffraction peaks of HP-NCA and SP-NCA are consistent with those of LiNiO2 (JCPDS no. 74-0919) with exception of a slight shift of peak position toward higher degree. The introduction of Co3+ and Al3+ which have smaller radiuses (0.545 Å and 0.535 Å) in replacement of Ni3+ with a greater radius (0.600 Å) is believed to be responsible for the slight shift of peak position.23,24 The clear separation of (006)/(102) and (018)/(110) peak pairs for HP-NCA and SP-NCA suggests the formation of highly ordered hexagonal layered structure. Furthermore, the intensity ratios of (003) and (104) peaks are bigger than 1.2, which indicates a low extent of cation mixing.25 Notably, the intensity ratio of (003)/(104) for HP-NCA is 1.46 while that for SP-NCA is 1.31, a result which indicates that HP-NCA has a lower cation mixing than SP-NCA. This result can be attributed to the fact that the porous microstructure of the heated precursor for HP-NCA is in favor of improving reaction activity among Al2O3, Li2O and the heated precursor during the calcining process.
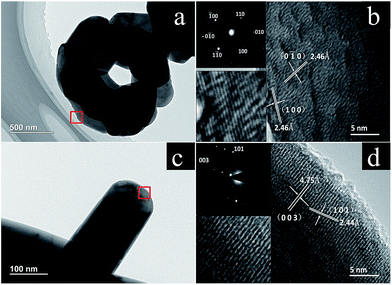
To further characterize the morphology and structure, TEM was conducted for HP-NCA and SP-NCA. Fig. 3a displays micro-sized particles made up of the primary plates, which is consistent with the SEM observation. As shown in Fig. 3b, there are two sets of lattice fringes with the same interplanar distance of 2.46 Å, corresponding to the (0![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) and (100) planes, respectively. The corresponding SAED pattern exhibits a hexagonal symmetry, indicating that these plates are single-crystalline. As illustrated in Fig. 3d, the lateral plane displays two sets of lattice fringes with the interplanar distances of 4.75 Å and 2.44 Å, corresponding to the (003) and (101) planes, respectively. These observations confirm that the frontal plane belongs to the electrochemically inert {001} facets and the lateral plane belongs to the electrochemically active {010} facets.15 As a contrast, five micro-regions at the edge of one small particle for SP-NCA were randomly selected to be characterized with HR-TEM. The corresponding images are shown in Fig. S3 (ESI†). The planes are (003), (101), (006) facets, respectively. No plane belonging to the electrochemically active {010} facets is observed. The observation is in accordance with the poorer rate capability of SP-NCA.
0) and (100) planes, respectively. The corresponding SAED pattern exhibits a hexagonal symmetry, indicating that these plates are single-crystalline. As illustrated in Fig. 3d, the lateral plane displays two sets of lattice fringes with the interplanar distances of 4.75 Å and 2.44 Å, corresponding to the (003) and (101) planes, respectively. These observations confirm that the frontal plane belongs to the electrochemically inert {001} facets and the lateral plane belongs to the electrochemically active {010} facets.15 As a contrast, five micro-regions at the edge of one small particle for SP-NCA were randomly selected to be characterized with HR-TEM. The corresponding images are shown in Fig. S3 (ESI†). The planes are (003), (101), (006) facets, respectively. No plane belonging to the electrochemically active {010} facets is observed. The observation is in accordance with the poorer rate capability of SP-NCA.
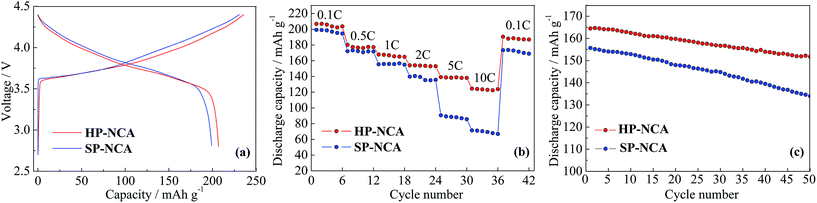
Electrochemical measurements were conducted to investigate the influence of morphology on electrochemical performances for HP-NCA and SP-NCA. Fig. 4a exhibits the initial charge/discharge curves of HP-NCA and SP-NCA at 0.1C (1C = 180 mA h g−1) in the voltage range of 2.8–4.4 V at room temperature. The initial discharge capacities of SP-NCA and HP-NCA are 199 and 207 mA h g−1, respectively, whilst the corresponding initial coulombic efficiencies are 84.3% and 88.6%, respectively. The larger discharge capacity and initial coulombic efficiency of HP-NCA can be ascribed to the lower cation mixing in the crystal of HP-NCA since a lower cation mixing was confirmed to ease the electrode polarization attributed to irreversible loss of capacity in previous literatures.26,27
To examine the rate capabilities of HP-NCA and SP-NCA, discharge capabilities were taken at 0.1, 0.5, 1.0, 2.0, 5.0, 10.0C in the voltage range of 2.8–4.4 V. As shown in Fig. 4b, the discharge capacities of SP-NCA are 199, 172, 155, 137, 89 and 70 mA h g−1 at different rates, respectively. As a sharp contrast, the discharge capacities of HP-NCA reach to 207, 179, 165, 154, 138 and 124 mA h g−1 at different rates, respectively. Moreover, the discharge capacity of HP-NCA is still as high as 193 mA h g−1 when the cell was re-cycled at 0.1C after cycling at 10.0C. The better rate capability of HP-NCA can be attributed to the previously mentioned observation that the microstructure with exposed {010} active planes facilitates Li+ rapid insertion/extraction.
The cyclic performances were obtained when cells were cycled for 50 cycles at 1.0C in the voltage range of 2.8–4.4 V. As shown in Fig. 4c, SP-NCA exhibits a rapid discharge fading from 156 to 134 mA h g−1 after 50 cycles and a low capacity retention of 85.9%. In contrast, HP-NCA shows a better cyclic performance with a slower discharge fading from 165 to 152 mA h g−1 after 50 cycles and a relatively high capacity retention of 92.1%.
Electrochemical impedance spectra (EIS) measurements for cells after 50 cycles were conducted to shed light on the better cyclic performance of HP-NCA. The Nyquist plots were obtained using cells cycled at the charge state of 4.4 V after 50 cycles at 1.0C and were shown in Fig. 5a. These Nyquist plots are made up of a high-frequency semicircle and a low-frequency oblique line, which were fitted using ZSimpWin software and an equivalent circuit illustrated in inset of Fig. 5a. In the equivalent circuit, Re represent the resistive contribution from electrolyte resistance and cell components. RSEI and CPEf are the resistance and capacitance of solid electrolyte interface (SEI) film, respectively. Rct and CPEdl represent the charge transfer resistance and associated double layer capacitance, respectively. Zw is the Warburg impedance.28 The fitting data are listed in Table S2 (ESI†). HP-NCA clearly shows a much smaller Rct than SP-NCA, a result which is in accord with the better cyclic performance of HP-NCA.
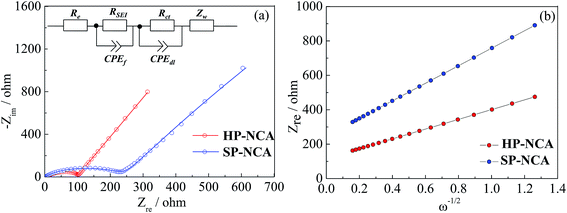 | ||
| Fig. 5 (a) Nyquist plots for HP-NCA and SP-NCA, inset shows a corresponding equivalent circuit. (b) The relation curves between Zre and ω−1/2 at low frequency for HP-NCA and SP-NCA. | ||
In order to verify that the microstructure with exposed {010} active planes facilitates Li+ rapid insertion/extraction, we also calculated Li+ diffusion coefficient (DLi+) according to the following equation:29
| D = R2T2F−4A−2C−2n−4σ−2/2 | (1) |
| Zre = Re + Rct + σω−1/2 | (2) |
Fig. 5b exhibits the relation curves between Zre and ω−1/2. Both curves display a linear characteristic. The calculated DLi+ values are listed in Table S2 (ESI†). The DLi+ value of HP-NCA is about four times larger than that of SP-NCA, a result which confirms that the microstructure with exposed {010} active planes of HP-NCA is conducive to the diffusion of Li+-ions.
Conclusion
In summary, hierarchical LiNi0.8Co0.15Al0.05O2 plates with exposed {010} active planes were successfully synthesized via a solvothermal method followed by solid-state reaction. The as-synthesized plates exhibit a high initial discharge capacity (207 mA h g−1 at 0.1C), an excellent rate capability (124 mA h g−1 at 10.0C) and a relatively good cyclic performance (92.1% capacity retention after 50 cycles at 1.0C). The calculated Li+ diffusion coefficient demonstrates that the microstructure with exposed {010} active planes for LiNi0.8Co0.15Al0.05O2 plates favors the Li+-ions diffusion and contributes to the improved rate capability.Acknowledgements
We are indebted to Margaret Yau for her kind help on language polishing.References
- T. Kim, J. Park, S. K. Chang, S. Choi, J. H. Ryu and H. Song, Adv. Energy Mater., 2012, 2, 860–872 CrossRef CAS.
- S. H. Ju, J. H. Kim and Y. C. Kang, Met. Mater. Int., 2010, 16, 299–303 CrossRef CAS.
- S. Y. Yang, X. Y. Wang and X. K. Yang, J. Solid State Electrochem., 2012, 16, 2823–2836 CrossRef CAS.
- H. Cao, B. J. Xia, Y. Zhang and N. X. Xu, Solid State Ionics, 2005, 176, 911–914 CrossRef CAS.
- H. Arai, S. Okada, Y. Sakurai and J. Yamaki, Solid State Ionics, 1998, 109, 295–302 CrossRef CAS.
- J. Shim, R. Kostecki, T. Richardson, X. Song and K. A. Striebel, J. Power Sources, 2002, 112, 222 CrossRef CAS.
- C. H. Chen, J. Liu, M. E. Stoll, G. Henriksen, D. R. Vissers and K. Amine, J. Power Sources, 2004, 128, 278 CrossRef CAS.
- C. J. Han, J. H. Yoon, W. I. Cho and H. Jang, J. Power Sources, 2004, 136, 132 CrossRef CAS.
- N. Machida, J. Kashiwagi, M. Naito and T. Shigematsu, Solid State Ionics, 2012, 225, 354–358 CrossRef CAS.
- Y. Xu, X. H. Li, Z. X. Wang, H. J. Guo and B. Huang, Mater. Lett., 2015, 143, 151–154 CrossRef CAS.
- Y. Cho, Y. S. Lee, A. A. Park, Y. Lee and J. Cho, Electrochim. Acta, 2010, 56, 333–339 CrossRef CAS.
- D. J. Lee, B. Scrosati and Y. K. Sun, J. Power Sources, 2011, 196, 7742–7746 CrossRef CAS.
- H. Z. Yang, P. X. Liu, Q. L. Chen, X. W. Liu, Y. W. Lu, S. F. Xie, L. Ni, X. Y. Wu, M. Y. Peng, Y. B. Chen, Y. F. Tang and Y. F. Chen, RSC Adv., 2014, 4, 35522–35530 RSC.
- N. T. Wu, H. Wu, W. Yuan, S. J. Liu, J. Y. Liao and Y. Zhang, J. Mater. Chem. A, 2015, 3, 13648–13655 CAS.
- F. Fu, G. L. Xu, Q. Wang, Y. P. Deng, X. Li, J. T. Li, L. Huang and S. G. Sun, J. Mater. Chem. A, 2013, 1, 3860–3864 CAS.
- Z. W. Guo, L. Xia, S. K. Fu, L. Huang, J. T. Li, Z. X. Wang, Z. Y. Zhou and S. G. Sun, Adv. Mater., 2010, 22, 4364–4367 CrossRef PubMed.
- J. L. Li, R. M. Yao and C. G. Cao, ACS Appl. Mater. Interfaces, 2014, 6, 5075–5082 CAS.
- L. Chen, Y. Su and S. Chen, Adv. Mater., 2014, 26, 6756–6760 CrossRef CAS PubMed.
- S. N. Lim, W. Ahn, S. Yeon and S. B. Park, Electrochim. Acta, 2014, 136, 1–9 CrossRef CAS.
- S. J. Zheng, R. Huang, Y. Makimura, Y. Ukyo, C. A. J. Fisher, T. Hirayama and Y. Ikuhara, J. Electrochem. Soc., 2011, 158, A357–A362 CrossRef CAS.
- D. Qian, B. Xu, H. M. Cho, T. Hatsukade, H. J. Carroll and Y. S. Meng, Chem. Mater., 2012, 24, 2744–2751 CrossRef CAS.
- S. R. Challa, A. T. Delariva, T. W. Hansen, S. Helveg, J. Sehested, P. L. Hansen, F. Garzon and A. K. Datye, J. Am. Chem. Soc., 2011, 133, 20672–20675 CrossRef CAS PubMed.
- H. Deng, I. Belharouak, H. Wu, D. Dambournet and K. Amine, J. Electrochem. Soc., 2010, 157, A776–A781 CrossRef CAS.
- J. H. Kim, C. W. Park and Y. K. Sun, Solid State Ionics, 2003, 164, 43–49 CrossRef CAS.
- Z. D. Huang, X. M. Liu, S. W. Oh, B. Zhang, P. C. Ma and J. K. Kim, J. Mater. Chem., 2011, 21, 10777–10784 RSC.
- K. M. Shaju, G. V. S. Rao and B. V. R. Chowdari, Electrochim. Acta, 2004, 49, 1565–1576 CrossRef CAS.
- J. P. Peres, C. Delmas, A. Rougier, M. Broussely, F. Perton, P. Biensan and P. Willmann, J. Phys. Chem. Solids, 1996, 57, 1057 CrossRef CAS.
- J. L. Li, C. B. Cao, X. Y. Xu, Y. Q. Zhu and R. M. Yao, J. Mater. Chem. A, 2013, 1, 11848–11852 CAS.
- Y. Xia, W. K. Zhang, H. Huang, Y. P. Gan, C. G. Li and X. Y. Tao, Mater. Sci. Eng., B, 2011, 176, 633–639 CrossRef CAS.
- C. Gao, H. Liu, G. B. Liu, J. Zhang and W. Wang, Mater. Sci. Eng., B, 2013, 178, 272–276 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and characterization details; Fig. S1–S3, Tables S1 and S2. See DOI: 10.1039/c6ra02694j |
| This journal is © The Royal Society of Chemistry 2016 |