Radical coupling polymerization (RCP) for synthesis of various polymers†
Abstract
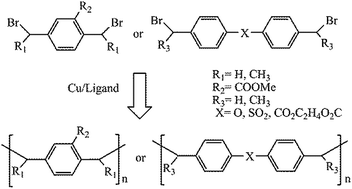
A general polymerization method is reported, which involves a direct radical coupling reaction of in situ formed benzyl-type biradicals from dibromide in the presence of a Cu(0)/ligand. The radical coupling polymerization can be employed to synthesize polyarene, polyester, polyether and polysulfone under mild conditions and within a short time.

 Please wait while we load your content...
Please wait while we load your content...