Bacterial siderophore mimicking iron complexes as DNA targeting antimicrobials†
Sunil Kumar Boda‡
a,
Subhendu Pandit‡a,
Aditya Garaib,
Debnath Palc and
Bikramjit Basu*ad
aLaboratory for Biomaterials – Materials Research Centre, Indian Institute of Science, Bangalore – 560012, India. E-mail: bikram@mrc.iisc.ernet.in
bDepartment of Inorganic and Physical Chemistry, Indian Institute of Science, Bangalore – 560012, India
cDepartment of Computational and Data Sciences, Indian Institute of Science, Bangalore – 560012, India
dCentre for Biosystems Science and Engineering, Indian Institute of Science, Bangalore – 560012, India
First published on 12th April 2016
Abstract
Microbial secretion of siderophores for iron uptake can be employed as an efficient strategy to smuggle in bactericidal agents by conjugation to iron. Three iron complexes: complex 1 [Fe(L1)2]Cl2 L1 = 3-(pyridin-2-yl)dipyrido[3,2-a:2′,3′-c]phenazine (pydppz or ligand 1), complex 2 [Fe(BHA)(L2)Cl]Cl·H20 (BHA = benzohydroxamate) L2 = pyrenyl-dipicolylamine (pydpa or ligand 2) and complex 3 [Fe(BHA)(L3)Cl]Cl·H20 L3 = phenyl-dipicolylamine (phdpa or ligand 3) were synthesized. The ligands were docked for binding to DNA and DNA polymerase I. The antibacterial efficacy of the iron complexes were evaluated against three pathogenic bacteria – Staphylococcus aureus (MRSA), Escherichia coli and Pseudomonas aeruginosa as well as cytotoxicity assessed in C2C12 mouse myoblast cells. In silico docking and molecular dynamic simulations of the ligands revealed stable and non-specific binding to DNA and DNA polymerase I, in the order: L1 > L2 > L3. The bactericidal effect of the iron complexes against MRSA predominantly occurred by bacterial DNA fragmentation as analyzed from gel electrophoresis and comet assay. The extent of DNA damage followed the order: complex 1 > complex 2 > complex 3, in commensurate with docking. Siderophore production elicited preferential bactericidal action of the iron complexes against MRSA. Both, complex 1 and complex 2 were 5–10 fold less toxic in C2C12 cells compared to MRSA. Taken together, a combination of DNA targeting and siderophore mimicking ligands conjugated to iron can be deployed as Trojan horse for the entry of antimicrobials into pathogenic bacteria. Thus, DNA targeting antimicrobials offer a promising solution to persistent bacterial infections. Their selectivity towards microbes can be promoted via siderophore uptake pathways.
1. Introduction
Iron, in its soluble form, is an essential nutrient for most bacteria. Iron is a co-factor for iron–sulphur clusters and haem proteins along with enzymes, like superoxide dismutase, peroxidase, glutamate synthase, and nitrogenase. These enzymes facilitate cellular processes in bacteria, such as oxidative phosphorylation, nitrogen fixation, electron transport and so on.1 Further, iron is a coenzyme or enzyme activator of ribonucleotide reductase, which converts ribonucleotides to deoxyribonucleotidides.2 However, most of the iron in aerobic environments exists as insoluble Fe-oxides or hydroxides. The solubility of ferric iron is 10−17 M at neutral pH, while bacteria require 10−5 to 10−7 M of iron for optimal growth.3 Hence, most pathogenic bacteria secrete high affinity Fe scavenging organic molecules called siderophores, which facilitate iron acquisition by forming soluble iron–chelate complexes. These iron–chelates are actively transported across the cell membrane and such transport is mediated by periplasmic binding proteins and cytoplasmic membrane transporters.4 Fe is a hard Lewis acid and hence most of the chelating siderophore molecules coordinate to ferric ions through Lewis bases with hard donor atoms, like neutral oxygen and nitrogen atoms or negatively charged oxygen. In general, three types of iron coordinating motifs are present in most microbial siderophores. These include hydroxyl groups from catechol moieties like in enterobactin secreted by E. coli, hydroxamate as in coprogen B and citrate derived α-hydroxy carboxylate motifs as in petrobactin or carboxylates in rhiozoferrin.5 The bidentate chelating siderophores have a greater affinity to ferric iron and form more stable hexacoordinate complexes, resulting from a smaller entropy change as compared to monodentate ligands. The secretion of siderophores under iron – deficient conditions is implicated in bacterial virulence and pathogenecity.6,7 Under infection of host by bacterial pathogens, the microbes secrete siderophore molecules and assimilate iron from mammalian iron-binding proteins, such as transferrin and lactoferrin.8One of the antimicrobial strategies being employed is the synthesis of artificial siderophores conjugated to antibiotics that are up taken by bacteria along with iron–chelates of the antibiotic-conjugated siderophores. Another less explored option is DNA targeting antimicrobials with specificity or selectivity for inducing bacteriotoxicity. The iron–chelates of siderophores can be used as ‘Trojan horse’ to circumvent permeability-mediated drug resistance and selective DNA targeting for bactericidal action against most pathogenic bacteria.9 In the present study, the iron complexes: complex 1 [Fe(L1)2]Cl2 L1 = 3-(pyridin-2-yl)dipyrido[3,2-a:2′,3′-c]phenazine (pydppz or ligand 1), complex 2 [Fe(BHA) (L2)Cl]Cl·H20 (BHA = benzohydroxamate) L2 = pyrenyl-dipicolylamine (pydpa or ligand 2), and complex 3 [Fe(BHA)(L3)Cl]Cl·H20 L3 = phenyl-dipicolylamine (phdpa or ligand 3), are tested for their antibacterial and antibiofilm efficacy against pathogenic Gram-positive bacteria – Staphylococcus aureus (MRSA, USA 300) and Gram-negative bacteria – E. coli K12 (MG 1655) wild type and Pseudomonas aeruginosa. Under similar dosage, the cytotoxicity of the above complexes is assessed in the dosage window of antimicrobial action using C2C12 mouse myoblast cells as a representative non-cancerous cell line. This is in the light of earlier studies reporting comparable photocytotoxicity elicited by identical iron complexes in HeLa cancer cells, MCF-7 breast cancer cells and human keratinocyte cells HaCaT (non-tumorogenic).10,11 One can, therefore, argue that such similar iron complexes could be potent bactericidal agents under non-photodynamic conditions, and presents a strong case for in silico and in vitro investigations of the bacteriotoxicity as envisaged in the current study. Hence, we have employed docking and molecular dynamic simulations to correlate in silico predictions and in vitro bacteriotoxicity results, in a unique approach to corroborate theory and experiments. Further, the therapeutic safety of such metal complexes as antibacterial agents is also assessed by TOPKAT, a computer assisted program that predicts chemical toxicity.12 Summarizing, we have deployed a novel approach via bacterial siderophore mimicking iron complexes as a Trojan horse strategy for bacterial uptake and eliciting bactericidal action via DNA intercalation and fragmentation.
2. Materials and methods
2.1 Ligand exchange of labile iron complexes
The three iron complexes were synthesized following earlier reported protocols.10,11 The detailed synthesis procedures are presented in the ESI† along with the reaction schemes and characterization of the ligands and complexes. Here, the labile nature of the synthesized iron complexes is characterized by ligand exchange reaction with a strong metal chelator 8-hydroxyquinoline (8-HQ).13 Incidentally, 8-HQ has been reported as a potential phytosiderophore.14 It is also known to be a chemical moiety present in quinolobactin, a natural siderophore produced by Pseudomonas fluorescens15 as well as an integral component of oxinobactin and sulfaxinobactin, both synthetic analogues of enterobactin siderophore secreted by E. coli.16 The ligand exchange of the iron complexes with an excess of 8-HQ was studied by monitoring the absorption spectral changes recorded using UV-vis spectrophotometer (Eppendorf). For the study, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 equivalents of 8-HQ
1 equivalents of 8-HQ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) complex 1 and 1
complex 1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 equivalents of 8-HQ
1 equivalents of 8-HQ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) complex 2/complex 3 in DMSO were mixed and the absorption spectra were recorded immediately and after a time interval. Further absorption spectra of Fe3+
complex 2/complex 3 in DMSO were mixed and the absorption spectra were recorded immediately and after a time interval. Further absorption spectra of Fe3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8-HQ in 1
8-HQ in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratios were recorded using 0.4 mM of Fe(NO3)3 as the iron precursor. These measurements were made to compare the absorption spectra of the iron complexes after ligand exchange with 8-HQ.
1 mole ratios were recorded using 0.4 mM of Fe(NO3)3 as the iron precursor. These measurements were made to compare the absorption spectra of the iron complexes after ligand exchange with 8-HQ.
2.2 DNA binding and fragmentation
Virtual screening of potential DNA and/or protein binding drug candidates has become a common and standard technology in modern drug discovery.17 The AutoDock18 and similar softwares have been widely used for virtual screening of such drug molecules. Here, we have used Autodock Vina19 and AutoDock tools17 to calculate and analyze the binding of the ligands of the labile iron complexes with few random nucleic acid sequences, B-DNA Dickerson dodecamer and DNA polymerase I. The molecular coordinates were taken from the Protein Data Bank (PDB; http://www.rcsb.org). Discovery Studio20 was used to visualize and generate graphical images of interactions. The default parameters were used for the molecular docking simulation. Binding energy, number of hydrogen bonds, number of electrostatic interaction and number of hydrophobic interaction in the docking mode with highest score has been taken as a measurement of theoretical binding strength. The experimental investigations of DNA binding and fragmentation were carried out by performing DNA gel electrophoresis and comet/single cell gel electrophoresis (SCGE) assays.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 5 min. The cell pellets were resuspended in 50 μL of lysostaphin (Sigma; 100 μg mL−1) and incubated at 37 °C for 10 min. Subsequently, 50 μL of proteinase K (100 μg mL−1) and 100 μL of 1× TE buffer (10 mM Tris–HCl of pH 8 and 0.1 mM EDTA) were added to each tube and incubated for an additional 10 min at 37 °C. Finally, the bacterial suspensions were placed in boiling water for 5 min to complete the lysis. 50 μL of the lysates were loaded into the wells of a 0.8% agarose gel and the samples were run at 5 V cm−1.
000 × g for 5 min. The cell pellets were resuspended in 50 μL of lysostaphin (Sigma; 100 μg mL−1) and incubated at 37 °C for 10 min. Subsequently, 50 μL of proteinase K (100 μg mL−1) and 100 μL of 1× TE buffer (10 mM Tris–HCl of pH 8 and 0.1 mM EDTA) were added to each tube and incubated for an additional 10 min at 37 °C. Finally, the bacterial suspensions were placed in boiling water for 5 min to complete the lysis. 50 μL of the lysates were loaded into the wells of a 0.8% agarose gel and the samples were run at 5 V cm−1.2.3 Antimicrobial and anti-biofilm testing
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 dilutions of the two dyes and incubated for 20 min, as optimized in our earlier studies.26,27 The samples were then washed twice with 1× PBS and were visualized under a fluorescence microscope (Nikon) with an oil immersion lens.
2000 dilutions of the two dyes and incubated for 20 min, as optimized in our earlier studies.26,27 The samples were then washed twice with 1× PBS and were visualized under a fluorescence microscope (Nikon) with an oil immersion lens.The bacteria in the logarithmic phase were cultured for 48 h in iron deficient MM9 media and supplemented with 3.13, 6.25, 12.5 and 25 μM concentrations of Fe2+ and iron complexes in a 96 well microtiter plate. It is understood that these are MIC/sub-MIC concentrations of the iron complexes can elicit siderophore production. After 48 h, the bacteria were centrifuged at 9600 × g and the supernatants were collected. 100 μL of the supernatant was treated with 100 μL of Chrome Azurol S (CAS) assay solution. The recipe and protocol for preparing the CAS assay solution was followed from a previous study.29 The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures of the culture supernatant and CAS assay solution were allowed to equilibrate in dark for 1 h at room temperature. The absorbance of the yellowish brown solution was measured at 630 nm in a multimode plate reader. Also, a wavelength scan of the absorbance spectra in the UV-visible range was performed in order to ascertain the type of Fe–siderophore species.
1 mixtures of the culture supernatant and CAS assay solution were allowed to equilibrate in dark for 1 h at room temperature. The absorbance of the yellowish brown solution was measured at 630 nm in a multimode plate reader. Also, a wavelength scan of the absorbance spectra in the UV-visible range was performed in order to ascertain the type of Fe–siderophore species.
2.4 Cytotoxicity assessment
2.5 Statistical analysis
The statistical analysis for all the presented data was carried out using IBM SPSS Statistics 20 software. All the experiments were carried out in triplicate and the analysis of variance (one way ANOVA) was adopted to determine the statistical significance between the iron complex treated bacteria/cells and the untreated controls. For the data analysis, Tukey and Games–Howell tests were employed to determine the statistical significance at p < 0.05, where p denotes the probability that there is no significant difference between the means.3. Results
We investigated the DNA binding affinities of the iron complex ligands in silico as well as antibacterial efficacy and cytotoxicity of the labile iron complexes in vitro. The degradation of bacterial DNA by the iron complexes is studied by gel electrophoresis methods. Further, the in vivo toxicity of the iron complexes is predicted by TOPKAT, in comparison to FDA approved drugs. Overall, the utility of in silico methods for predicting chemical and molecular toxicity are demonstrated in the present study.3.1 Labile behavior of the iron complexes
The molecular structures of the iron complexes and their corresponding ligands are shown in Fig. 1. The labile behavior of the iron complexes is established by ligand exchange reaction with 8-hydroxyquinoline (8-HQ), a strong metal chelator. Following the addition of excess of 8-HQ, an instantaneous color change was observed for both complex 2 and 3. The change, as recorded in the absorption spectra, indicated a rapid ligand exchange reaction. However, in case of complex 1, we observed a continuous decrease in the intensity of the absorption peak in the UV range. The addition of few drops of hydrogen peroxide (H2O2) accelerated the ligand exchange reaction, resulting in a quick change in the absorption spectra (see ESI Fig. S2A†). The ligand exchange reactions of complex 2 and complex 3 with 8-HQ are shown in Fig. S2B and C,† respectively. A clear correlation of the absorption spectra of the iron complexes, following ligand exchange with 8-HQ (Fig. S2A–C†) can be observed by comparison with the UV-visible absorption spectra for iron complexes prepared using ferric iron![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8-HQ in 1
8-HQ in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratios (Fig. S2D†). The absorption maxima at 450 nm and near 600 nm recorded for complex 1, 2 and 3 after ligand exchange with 8-HQ match well with the ferric iron
1 mole ratios (Fig. S2D†). The absorption maxima at 450 nm and near 600 nm recorded for complex 1, 2 and 3 after ligand exchange with 8-HQ match well with the ferric iron![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8-HQ in different mole ratios. The above results qualitatively proves the labile nature of the three iron complexes used further for molecular docking and elucidating the bacterial/cell cytotoxicity, as described in the subsequent sections.
8-HQ in different mole ratios. The above results qualitatively proves the labile nature of the three iron complexes used further for molecular docking and elucidating the bacterial/cell cytotoxicity, as described in the subsequent sections.
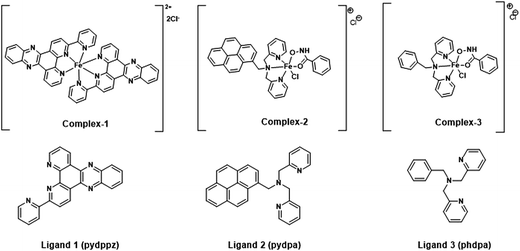 | ||
| Fig. 1 Molecular structures of the siderophore mimicking iron complexes and associated ligands synthesized for the study. | ||
3.2 In silico and in vitro analysis of DNA binding and fragmentation
The docking results of the ligands from the labile iron complexes have been presented in terms of the binding energies and number of interactions between the ligand and the receptor sequence of DNA/protein. The best binding modes and binding stability of the ligands to DNA and DNA Pol I were determined by docking studies and molecular dynamic simulations.Binding energy is a better parameter to determine the strength of interaction compared to counting number of interactions, because there may be a handful of weak interactions of very low significance, though the total interaction is not very strong. From the best five docking mode energies of ligand 1, 2 and 3 binding to Dickerson dodecamer (shown in ESI Table S2†), we can see the energies are very closely spaced. This suggests that there is no preferential site and the ligands bind to DNA in a non-specific manner. The representative graphical image of interaction between Dickerson dodecamer (PDB ID: 1BNA) and ligand 1 is shown in Fig. 2A(a) while those with ligand 2 and ligand 3 are shown in Fig. S3.† All such observations corroborate well with the fact that the ligands bind to the DNA groove, as experimentally observed by Garai et al.10 Further, the binding constants (Kb) for the intercalation of the iron complexes with calf-thymus DNA (determined by UV-vis absorption titrations) have been listed in Table 5. In the present work, we confirm that the previously reported experimental data10,11 (Table 5) are in excellent agreement with the docking results of specific and randomly designed DNA sequences with the aromatic ligands of the iron complexes (Tables 1 and S1†).
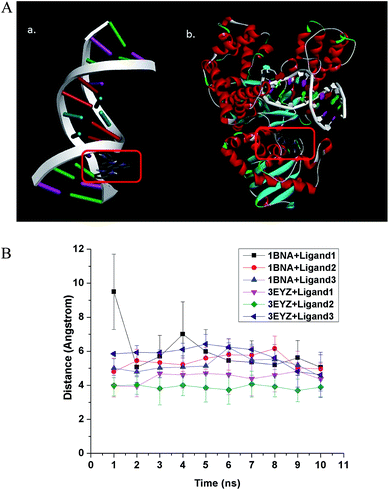 | ||
| Fig. 2 (A) Interaction of ligand 1 with (a) B-DNA Dickerson dodecamer (1BNA) and (b) DNA Pol I (3EYZ). All the three ligands bind to the minor groove of DNA in a non-specific manner. For DNA Pol I, ligand 1 bind to the active site specifically, ligand 2 exhibits moderate specificity and ligand 3 has a non-specific binding to the active site. The binding is stable in all cases irrespective of the specificity, as confirmed by the molecular dynamics calculations. (B) Average distance between centroid of the ligand and three randomly chosen atoms at the binding site of the receptor molecule has been calculated in an interval of 1 ns of the 10 ns molecular dynamics (MD) calculation. The average distance between centroid of ligand and randomly chosen atoms at the binding site with time has been plotted. It shows stable binding of the ligands to DNA (1BNA)/DNA Pol I (3EYZ). | ||
The best ligand binding modes were subjected to 10 ns molecular dynamics simulation to confirm the stability of the complexes. Total energy versus time plot (ESI Fig. S4†) suggests stable binding. We plotted distance between randomly chosen atoms of the ligand and binding site with time, and the plot also indicates stable binding (Fig. 2B). The ligands bind to DNA in a non-specific manner and such binding is stable, as suggested by the molecular dynamics simulation.
| Ligand | Binding energy for DNA Pol I (3EYZ) (kcal mol−1) | Types and no. of ligand–DNA Pol I interactions | |||
|---|---|---|---|---|---|
| H-bond (conventional) | Electrostatic | Hydrophobic | Total | ||
| Ligand 1 | 10.6 | 4 | 2 | 0 | 6 |
| Ligand 2 | 9.6 | 2 | 6 | 0 | 8 |
| Ligand 3 | 6.4 | 2 | 2 | 0 | 4 |
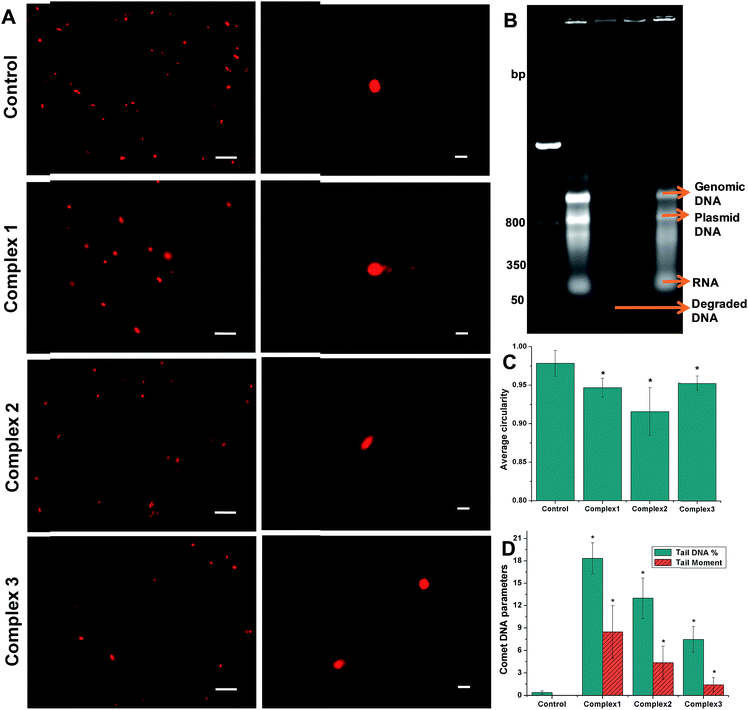
Comet assay or SCGE was carried out to ascertain damage to the bacterial genomic DNA by the iron complex ligands at lower dosage of 10 μM. Fig. 3A present low and high magnification images of control and iron complex treated bacterial DNA captured under a fluorescence and confocal microscope respectively. Using the image analysis tools provided by Image J software, the average circularity of bacterial DNA was determined from a minimum of n = 6 images, excluding the blotched signals. It can be clearly seen from Fig. 3C that average circularity of the DNA decreased from spherical in the control towards convex/ellipsoid shape in the iron complex treated samples. Further analysis of the DNA comets was performed with the help of the OpenComet plugin in Image J. An automated scoring and analysis of the comets was carried out following the established guidelines.34 The parameters used to estimate DNA damage were the tail DNA% and tail moment of the comets. Fig. 3D shows that the DNA damage parameters of the iron complex treated bacteria were significantly greater than their respective control parameters. Importantly, the order of DNA strand breakage is commensurate with the order of DNA binding affinities of the iron complex ligands.
3.3 Antimicrobial testing of iron complexes
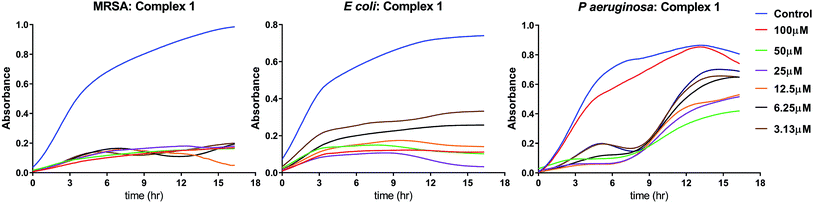
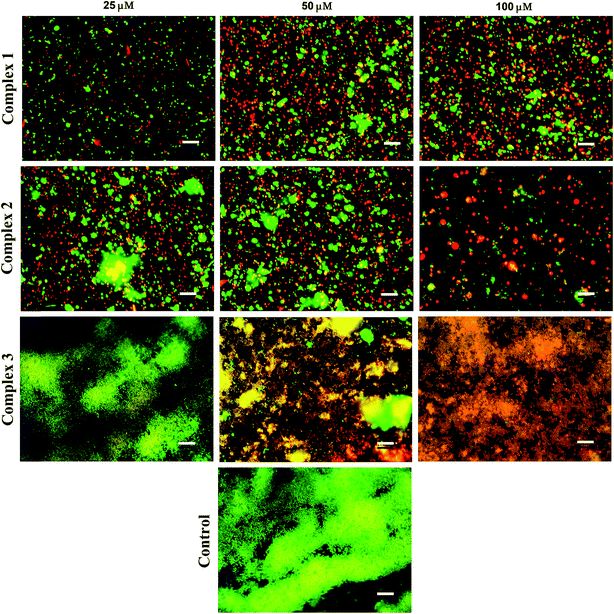
The iron complexes 1, 2 and 3 were tested against Gram positive S. aureus and Gram-negative E. coli and P. aeruginosa, for their antibacterial and anti-biofilm activity. All the protocols used for the testing of the iron complexes are in consonance with standard microbial practices and methods, as mentioned in the guidelines by the National Committee for Clinical Laboratory Standards (NCCLS).35| Complex | MIC (μM) of iron complexes against pathogenic bacteria | ||
|---|---|---|---|
| S. aureus (MRSA) | E. coli | P. aeruginosa | |
| Complex 1 | 3.13 | >100 | >100 |
| Complex 2 | 25 | >100 | >100 |
| Complex 3 | >100 | >100 | >100 |
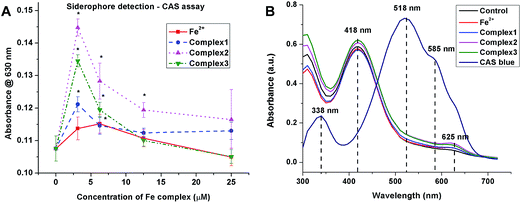
Furthermore, the UV-visible absorption spectra of the CAS solution and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of culture supernatants and CAS shuttle solutions show marked differences in the absorption maxima (Fig. 7B). The absorption spectrum of the CAS blue assay solution exhibited absorption maxima at 338 nm and 518 nm along with a shoulder peak at 585 nm. In contrast, the 1
1 mixture of culture supernatants and CAS shuttle solutions show marked differences in the absorption maxima (Fig. 7B). The absorption spectrum of the CAS blue assay solution exhibited absorption maxima at 338 nm and 518 nm along with a shoulder peak at 585 nm. In contrast, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 equilibrated mixtures of CAS blue and culture supernatants containing siderophores exhibited absorption maximum at 418 nm, corresponding to Fe–CAS red complex and another low intensity absorption maximum at 625 nm due to Fe–siderophore complex. The UV-visible absorption bands for the three iron complexes (1, 2 and 3) were however not detected. This suggests that the labile iron complexes have apparently undergone complete ligand exchange with the siderophore molecules and transported into the bacterial cells via membrane receptors.
1 equilibrated mixtures of CAS blue and culture supernatants containing siderophores exhibited absorption maximum at 418 nm, corresponding to Fe–CAS red complex and another low intensity absorption maximum at 625 nm due to Fe–siderophore complex. The UV-visible absorption bands for the three iron complexes (1, 2 and 3) were however not detected. This suggests that the labile iron complexes have apparently undergone complete ligand exchange with the siderophore molecules and transported into the bacterial cells via membrane receptors.
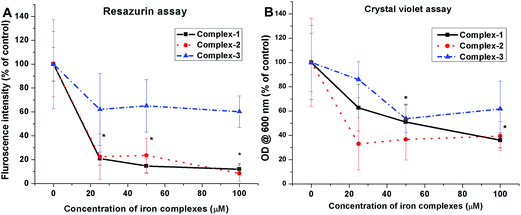
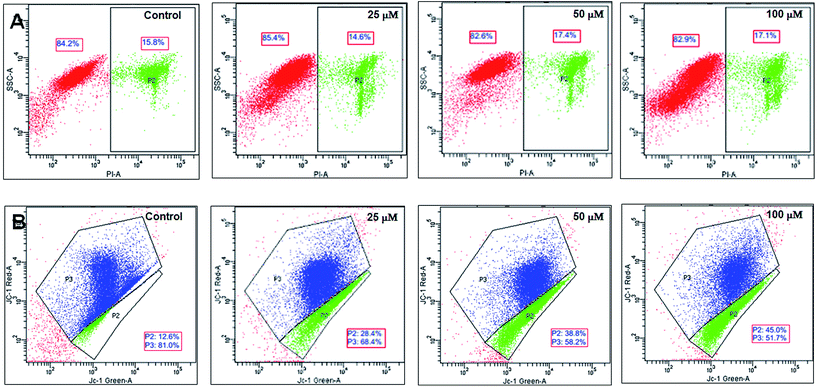
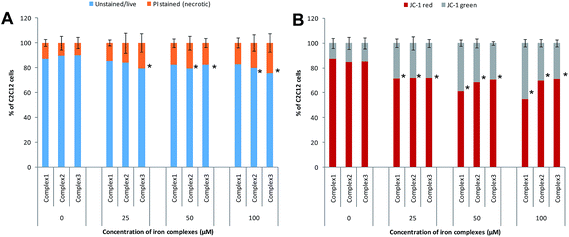
3.4 Cell cytotoxicity assessment
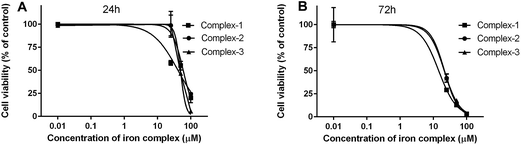
In a manner similar to the treatment of suspension cultures and bacterial biofilms, cell monolayers of C2C12 mouse myoblasts were treated with 25, 50 and 100 μM concentrations of the iron complexes. The results were analyzed using MTT assay and flow cytometry.| Complex | IC50 (μM) of iron complexes against C2C12 mouse myoblasts | |
|---|---|---|
| 24 h | 72 h | |
| Complex 1 | 41.3 ± 1.1 | 14.5 ± 1.3 |
| Complex 2 | 59.1 ± 1.1 | 21.6 ± 1.0 |
| Complex 3 | 48.9 ± 1.0 | 21.4 ± 1.0 |
| Complex | Equilibrium binding constants (Kb/M−1) determined from UV-vis absorption titrations |
|---|---|
| Complex 1 | 3.4 (±0.6) × 106 |
| Complex 2 | 6.4 (±0.8) × 105 |
| Complex 3 | 2.6 (±0.5) × 105 |
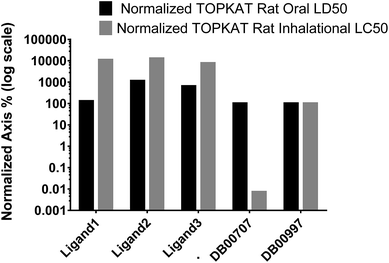
| Molecule/drug | Rat_inhalational_LC50 (mg m−3 h−1) | Rat_oral_LD50 (g kg−1 of body weight) | ||
|---|---|---|---|---|
| TOPKAT | Experimental | TOPKAT | Experimental | |
| a ND – not determined, NA – not applicable, * information from material safety datasheets MSDS. | ||||
| Ligand 1 | 5985.149 | ND | 0.29 | ND |
| Ligand 2 | 7017.849 | ND | 2.583 | ND |
| Ligand 3 | 4218.606 | ND | 1.461 | ND |
| Porfimer (DB00707) | 0.004 | NA | 0.227 | 0.065* |
| Doxorubicin (DB00997) | 55.258 | NA | 0.227 | 0.570–0.698* |
4. Discussion
4.1 Iron complexes elicit bactericidal action by siderophore mediated uptake and DNA fragmentation
Iron is an essential micronutrient for bacteria to replicate and cause infection in vertebrate hosts. Recent works have shown that iron is critical for biofilm formation by bacteria on solid surfaces.38,39 It was elucidated that iron chelators in culture media prevented S. aureus attachment onto material surfaces and reduced polysaccharide intercellular adhesion (PIA) secretion.39 In another study, the addition of gallium (Ga) to the culture media was shown to prevent biofilm formation in Pseudomonas aeruginosa. The chemical similarity of Ga to Fe supposedly interfered in Fe uptake and signaling leading to the anti-biofilm effect.38 The addition of exogenous iron effect reversed the effect in both cases. Under iron starvation, bacteria are reported to secrete iron chelating molecules called siderophores. Based on the chemical moieties that coordinate to iron, siderophores are broadly classified into cateocholate and hydroxamate types.40 There can be two possible antimicrobial strategies based on siderophore production and iron transport: (i) the synthesis of iron chelating molecules with strong binding affinity to iron and thus deprive bacteria with essential iron necessary for biofilm formation. (ii) Siderophore transport also makes such iron chelate producing bacteria more susceptible to the entry of lethal molecules/drugs and antimicrobial payloads conjugated to labile iron complexes.In the present study, the latter of the above two strategies has been applied successfully against MRSA in planktonic and biofilm cultures. The iron complexes containing identical hydroxamate siderophore moieties and/or different DNA intercalators were 5–10 times less toxic in C2C12 cells as compared to MRSA. Especially in the case of complex 1, a MIC value of ∼3 μM was recorded against MRSA while the IC50 doses against C2C12 cells were ∼40 μM and ∼15 μM after 24 h and 48 h of treatment, respectively. These results are promising in the light of earlier works showing similar photocytotoxicity of the iron complexes in both cancerous and non-cancerous cell lines.10,11 The mechanism of bactericidal action involves siderophore mediated uptake of iron complexes and bacterial DNA fragmentation as testified by gel electrophoresis and comet assay (Fig. 3). A high dose of 100 μM caused complete degradation of both genomic and plasmid DNA by complex 1, while a much lower concentration of 10 μM led to the formation of the formation of distinct comet tails. A comparison of the DNA fragmentation induced by the iron complexes is shown in Fig. 3D.
4.2 DNA as the primary target for the development of next generation antimicrobials
In the light of the emergence of multidrug bacterial resistance, DNA is of late being explored as the primary target in the development of antibacterial drugs.41 In this regard, a host of DNA groove binding molecules in the form of transition metal complexes with aromatic π-acceptor ligands are also being developed.42 Such metal complexes can interact with DNA by – (i) direct metal atom/ion coordination to the DNA bases, (ii) non-covalently by van der Waals or π-interactions of the aromatic residues with DNA, (iii) through heteroatoms such as O, N and S by hydrogen bonding between the heterocyclic aromatic ligands and DNA.43 In the current study, the antimicrobial potential of iron complexes comprising of DNA intercalating ligands has been successfully elucidated against Gram-positive MRSA. Further, an excellent correlation between in silico docking predictions and in vitro antibacterial results arising from bacterial DNA fragmentation is evident from the data presented in Tables 1–3.However, one of the problems posed by DNA groove binding molecules is their potential host cytotoxicity and lack of specificity/selectivity to the target organisms. One of the methods of achieving selectivity towards bacterial pathogens is the conjugation of antimicrobials/antibiotics to siderophores.44 In the present study, the cytotoxicity of the iron complexes towards mammalian cells have been circumvented considerably by the design of the iron complexes sporting benzhydroxamate ligands. These ligands have a structural similarity to the hydroxamate class of bacterial siderophores, which promoted greater bactericidal than cytocidal action, as suggested by the data in Tables 3 and 4 Further, TOPKAT in vivo toxicity analysis of our iron complexes predicts them to be comparable to FDA approved drug molecules (Table 6). Taken together, we demonstrate in this study the design of iron complexes conjugated to DNA intercalators and siderophore mimicking chelators as a potent strategy for the development of DNA targeting antimicrobial agents.
5. Conclusions
The design of labile iron complexes with a combination of ligands, which possess structural similarity to siderophores for bacterial uptake and those eliciting bactericidal action by DNA intercalation and fragmentation is demonstrated as an efficient strategy to overcome the pathogenic MRSA in the present work. Such iron complexes are 5–10 fold less toxic in C2C12 mouse myoblasts and this presents a therapeutic dosage window for their application as DNA targeting antimicrobial agents. Further, TOPKAT in vivo toxicity predictions confirm the therapeutic safety of the complexes in comparison to representative FDA approved drugs. The experimental bacteriotoxicity and bacterial DNA fragmentation elicited by the iron complexes are commensurate with the molecular docking determined binding affinities of the ligands to DNA and DNA polymerase I. Thus, DNA targeting in conjunction with siderophore mimics can be deployed as an efficient antimicrobial strategy to address persistent bacterial infections.Acknowledgements
The authors acknowledge the major funding support from the Department of Science and Technology (DST) and Department of Biotechnology (DBT), Govt. of India under ‘Centers of Excellence and Innovation in Biotechnology’ scheme through the center of excellence project – 'Translational Center on Biomaterials for Orthopedic and Dental Applications'. Prof. A. R. Chakravarty from Department of Inorganic and Physical Chemistry, IISc is acknowledged for his guidance and suggestion for synthesis and characterization of the complexes used in this study. Supercomputer Education and Research Centre (SERC), IISc is deeply acknowledged for providing the necessary software and computational tools for performing the Docking and Molecular Dynamics studies. The authors thank the IISc FACS facility for helping with the flow cytometry experiments. One of the authors, Sunil Kumar B. (09/079 (2501)/2011-EMR-I dt. 16-11-2011) acknowledges the Council for Scientific and Industrial Research (CSIR) for providing scholarship during the period of study and Subhendu Pandit acknowledges Department of Science and Technology (DST) for his KVPY undergraduate scholarship.References
- A. J. M. Messenger and R. Barclay, Bacteria, iron and pathogenicity, Biochem. Educ., 1983, 11(2), 54–63 CrossRef CAS.
- A. Symeonidis and M. Marangos, Iron and Microbial Growth, Insight and Control of Infectious Disease in Global Scenario, 2012, ISBN 978-953-51-0319-6 Search PubMed.
- S. C. Andrews, A. K. Robinson and F. Rodriguez-Quinones, Bacterial iron homeostasis, FEMS Microbiol. Rev., 2003, 27(2–3), 215–237 CrossRef CAS PubMed.
- J. S. Brown and D. W. Holden, Iron acquisition by Gram-positive bacterial pathogens, Microbes Infect., 2002, 4(11), 1149–1156 CrossRef CAS PubMed.
- F. C. Beasley and D. E. Heinrichs, Siderophore-mediated iron acquisition in the Staphylococci, J. Inorg. Biochem., 2010, 104(3), 282–288 CrossRef CAS PubMed.
- M. Miethke and M. A. Marahiel, Siderophore-based iron acquisition and pathogen control, Microbiol. Mol. Biol. Rev., 2007, 71(3), 413–451 CrossRef CAS PubMed.
- B. Schwyn and J. B. Neilands, Anal. Biochem, 1987, 160, 47–56 CrossRef CAS PubMed.
- C. Ratledge and L. G. Dover, Iron metabolism in pathogenic bacteria, Annu. Rev. Microbiol., 2000, 54, 881–941 CrossRef CAS PubMed.
- C. Ji, P. A. Miller and M. J. Miller, Iron Transport-Mediated Drug Delivery: Practical Syntheses and In Vitro Antibacterial Studies of Tris-Catecholate Siderophore-Aminopenicillin Conjugates Reveals Selectively Potent Antipseudomonal Activity, J. Am. Chem. Soc., 2012, 134(24), 9898–9901 CrossRef CAS PubMed.
- A. Garai, U. Basu, I. Khan, I. Pant, A. Hussain, P. Kondaiah and A. R. Chakravarty, Iron(III) benzhydroxamates of dipicolylamines for photocytotoxicity in red light and cellular imaging, Polyhedron, 2014, 73, 124–132 CrossRef CAS.
- A. Garai, U. Basu, I. L. A. Pant, P. Kondaiah and A. R. Chakravarty, Polypyridyl iron(II) complexes showing remarkable photocytotoxicity in visible light, J. Chem. Sci., 2015, 127(4), 609–618 CrossRef CAS.
- N. F. Cariello, J. D. Wilson, B. H. Britt, D. J. Wedd, B. Burlinson and V. Gombar, Comparison of the computer programs DEREK and TOPKAT to predict bacterial mutagenicity. Deductive Estimate of Risk from Existing Knowledge. Toxicity Prediction by Komputer Assisted Technology, Mutagenesis, 2002, 17(4), 321–329 CrossRef CAS PubMed.
- V. Prachayasittikul, S. Prachayasittikul, S. Ruchirawat and V. Prachayasittikul, 8-Hydroxyquinolines: a review of their metal chelating properties and medicinal applications, Drug Des., Dev. Ther., 2013, 7, 1157–1178 CrossRef PubMed.
- R. K. Tewari, G. Bachmann and F. Hadacek, Iron in complex with the alleged phytosiderophore 8-hydroxyquinoline induces functional iron deficiency and non-autolytic programmed cell death in rapeseed plants, Environ. Exp. Bot., 2015, 109, 151–160 CrossRef CAS.
- D. Mossialos, J.-M. Meyer, H. Budzikiewicz, U. Wolff, N. Koedam, C. Baysse, V. Anjaiah and P. Cornelis, Quinolobactin, a New Siderophore of Pseudomonas fluorescens ATCC 17400, the Production of Which Is Repressed by the Cognate Pyoverdine, Appl. Environ. Microbiol., 2000, 66(2), 487–492 CrossRef CAS PubMed.
- A. du Moulinet d'Hardemare, G. Gellon, C. Philouze and G. Serratrice, Oxinobactin and sulfoxinobactin, abiotic siderophore analogues to enterobactin involving 8-hydroxyquinoline subunits: thermodynamic and structural studies, Inorg. Chem., 2012, 51(22), 12142–12151 CrossRef PubMed.
- D. Seeliger and B. L. de Groot, Ligand docking and binding site analysis with PyMOL and Autodock/Vina, J. Comput.-Aided Mol. Des., 2010, 24(5), 417–422 CrossRef CAS PubMed.
- G. M. Morris, R. Huey, W. Lindstrom, M. F. Sanner, R. K. Belew, D. S. Goodsell and A. J. Olson, AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility, J. Comput. Chem., 2009, 30(16), 2785–2791 CrossRef CAS PubMed.
- O. Trott and A. J. Olson, AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading, J. Comput. Chem., 2009, 31(2), 455–461 Search PubMed.
- Accelrys Software Inc., Discovery Studio Modeling Environment, Release, 40, 2013.
- H. R. Drew, S. Samson and R. E. Dickerson, Structure of a B-DNA dodecamer at 16 K, PNAS, 1982, 79(13), 4040–4044 CrossRef CAS.
- A. A. Golosov, J. J. Warren, L. S. Beese and M. Karplus, The mechanism of the translocation step in DNA replication by DNA polymerase I: a computer simulation analysis, Structure, 2010, 18(1), 83–93 CrossRef CAS PubMed.
- S. Unal, J. Hoskins, J. E. Flokowitsch, C. Y. Wu, D. A. Preston and P. L. Skatrud, Detection of methicillin-resistant Staphylococci by using the polymerase chain reaction, J. Clin. Microbiol., 1992, 30(7), 1685–1691 CAS.
- V. Didenko and P. Olive, The Comet Assay, in In Situ Detection of DNA Damage, Humana Press, 2002, vol. 203, pp. 179–194 Search PubMed.
- S. K. Boda, J. Broda, F. Schiefer, J. Weber-Heynemann, M. Hoss, U. Simon, B. Basu and W. Jahnen-Dechent, Cytotoxicity of Ultrasmall Gold Nanoparticles on Planktonic and Biofilm Encapsulated Gram-Positive Staphylococci, Small, 2015, 11(26), 3183–3193 CrossRef CAS PubMed.
- S. K. Boda, I. Bajpai and B. Basu, Inhibitory effect of direct electric field and HA-ZnO composites on S. aureus biofilm formation, J. Biomed. Mater. Res., Part B, 2015 DOI:10.1002/jbm.b.33455.
- S. K. Boda, K. Ravikumar, D. K. Saini and B. Basu, Differential viability response of prokaryotes and eukaryotes to high strength pulsed magnetic stimuli, Bioelectrochemistry, 2015, 106, 276–289 CrossRef CAS PubMed.
- R. M. Murugappan, A. Aravinth, R. Rajaroobia, M. Karthikeyan and M. R. Alamelu, Optimization of MM9 Medium Constituents for Enhancement of Siderophoregenesis in Marine Pseudomonas putida Using Response Surface Methodology, Indian J. Microbiol., 2012, 52(3), 433–441 CrossRef CAS PubMed.
- D. B. Alexander and D. A. Zuberer, Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria, Biol. Fertil. Soils, 1991, 12(1), 39–45 CrossRef CAS.
- M. Reers, T. W. Smith and L. B. Chen, J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential, Biochemistry, 1991, 30(18), 4480–4486 CrossRef CAS PubMed.
- S. T. Smiley, M. Reers, C. Mottola-Hartshorn, M. Lin, A. Chen, T. W. Smith, G. D. Steele Jr and L. B. Chen, Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1, PNAS, 1991, 88(9), 3671–3675 CrossRef CAS.
- A. Amini, S. H. Muggleton, H. Lodhi and M. J. E. Sternberg, A Novel Logic-Based Approach for Quantitative Toxicology Prediction, J. Chem. Inf. Model., 2007, 47(3), 998–1006 CrossRef CAS PubMed.
- V. Law, C. Knox, Y. Djoumbou, T. Jewison, A. C. Guo, Y. Liu, A. Maciejewski, D. Arndt, M. Wilson and V. Neveu, et al., DrugBank 4.0: shedding new light on drug metabolism, Nucleic Acids Res., 2014, 42, D1091–D1097 CrossRef CAS PubMed.
- B. M. Gyori, G. Venkatachalam, P. S. Thiagarajan, D. Hsu and M.-V. Clement, OpenComet: An automated tool for comet assay image analysis, Redox Biol., 2014, 2, 457–465 CrossRef CAS PubMed.
- C. C. Ginocchio, Role of NCCLS in antimicrobial susceptibility testing and monitoring, Am. J. Health-Syst. Pharm., 2002, 59(3), S7–S11 Search PubMed.
- E. V. Sorokina, T. P. Yudina, I. A. Bubnov and V. S. Danilov, Assessment of iron toxicity using a luminescent bacterial test with an Escherichia coli recombinant strain, Microbiology, 2013, 82(4), 439–444 CAS.
- R. Z. Sayyed, M. D. Badgujar, H. M. Sonwane, M. M. Mhaske and S. B. Chincholkar, Production of microbial iron chelators (siderophores) by fluorescent Pseudomonads, J. Ind. Microbiol. Biotechnol., 2005, 4, 486–490 Search PubMed.
- Y. Kaneko, M. Thoendel, O. Olakanmi, B. E. Britigan and P. K. Singh, The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity, J. Clin. Invest., 2007, 117(4), 877–888 CrossRef CAS PubMed.
- M.-H. Lin, J.-C. Shu, H.-Y. Huang and Y.-C. Cheng, Involvement of Iron in Biofilm Formation by Staphylococcus aureus, PLoS One, 2012, 7(3), e34388 CAS.
- J. B. Neilands, Methodology of siderophores, in Siderophores from Microorganisms and Plants, Springer, Berlin Heidelberg, 1984, vol. 58, pp. 1–24 Search PubMed.
- A. Bolhuis and J. R. Aldrich-Wright, DNA as a target for antimicrobials, Bioorg. Chem., 2014, 55, 51–59 CrossRef CAS PubMed.
- H.-K. Liu and P. J. Sadler, Metal Complexes as DNA Intercalators, Acc. Chem. Res., 2011, 44(5), 349–359 CrossRef CAS PubMed.
- I. Mames, A. Rodger and J. Kowalski, Tetraaza[14]macrocyclic Transition Metal Complexes as DNA Intercalators, Eur. J. Inorg. Chem., 2015, 630–639 CrossRef CAS.
- T. A. Wencewicz, T. E. Long, U. Mollmann and M. J. Miller, Trihydroxamate Siderophore-Fluoroquinolone Conjugates Are Selective Sideromycin Antibiotics that Target Staphylococcus aureus, Bioconjugate Chem., 2013, 24(3), 473–486 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c6ra02603f |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2016 |