Effect of cerous phosphates with different crystal structures on their acidity and catalytic activity for the dehydration of glucose into 5-(hydroxymethyl)furfural†
Lina Wang,
Fulong Yuan,
Xiaoyu Niu,
Chuanhong Kang,
Pengying Li,
Zhibin Li* and
Yujun Zhu*
Key Laboratory of Functional Inorganic Material Chemistry (Heilongjiang University), Ministry of Education, School of Chemistry and Materials, Heilongjiang University, Harbin, 150080, P. R. China. E-mail: yujunzhu@hlju.edu.cn; lizb@hlju.edu.cn; Fax: +86-451-86609650; Tel: +86-451-86609650
First published on 7th April 2016
Abstract
A series of cerous phosphate (CP) catalysts with different crystal structures were synthesized by a hydrothermal method at different temperatures (120, 140, 160, 180 and 200 °C) and their performances for the dehydration of glucose into 5-(hydroxymethyl)furfural (HMF) were also thoroughly investigated. These catalysts were characterized by XRD, N2 adsorption–desorption, SEM, in situ DRIFT, NH3-TPD and XPS. The results indicate that changing the temperature of synthesis will lead to a transformation of the crystal phase and morphology from 120 °C (nanoparticles, hexagonal structure) to 200 °C (nanorods, monoclinic structure); also, the different crystal phases possess different surface Ce4+ amounts and acidities. A good linear correlation is found between the Lewis acid content and the surface Ce4+ amount among these CP catalysts, and very good linearity is also displayed between the Lewis acid amount and the conversion of glucose or the selectivity of HMF, which indicates that Lewis acidity plays an important role in the dehydration of glucose to HMF. CP120, which has a hexagonal crystal structure, exhibits the best catalytic activity (97% conversion of glucose and 61% yield of HMF) because it has the highest amounts of Lewis acid and total acid.
Introduction
Due to the decline of fossil resources and concerns regarding CO2 emissions, the utilization of renewable sources such as solar, nuclear, and wind energies and biomass resources has attracted much interest.1–4 Biomasses consisting of cellulose, lignose, starch, glucose and fructose have been widely studied due to their advantages of wide distribution, huge production, renewability, lack of pollution5,6 and prospective application values.7,8 5-(Hydroxymethyl)furfural (HMF), a green platform molecule derived from biomass, is recognized as a feedstock for the production of advanced chemicals and has been applied in numerous industrial processes. For instance, 2,5-dimethylfuran produced from the catalytic hydrogenation of 5-HMF can be used as an additive in automotive liquid biofuels. 2,5-Furandicarboxylic acid from the oxidation of 5-HMF is also considered to be an important and valuable chemical as a substitute of terephthalic acid.9Presently, HMF is mainly produced from the catalytic dehydration of fructose and glucose.1–5,10,11 High yield HMF transformation of fructose has been reported in aqueous phase, organic solvent and aqueous/organic mixtures.2,10 Unfortunately, the transformation of fructose is not suitable for large industrial applications due to its high cost even with a superior yield of HMF.12,13 Glucose offers another alternative, as it is inexpensive and easy to obtain; glucose can be first converted to fructose through an isomerization process,11,14 followed by conversion to HMF by a dehydration process (Scheme 1).15
Many attempts have been made to find better catalysts for this reaction,16 such as H2SO4,11 HCl,17 metallic oxide14 and chromium chloride;18 however, inorganic liquid acid catalysts (H2SO4 and HCl) led to an increase of humin formation from the direct dehydration of glucose and a lower selectivity for HMF.11,17 In recent years, solid acid catalysts have been applied in the catalytic transformation of glucose to HMF. In a study by Zhang and co-workers, amorphous catalysts of Cr2O3, SnO2, SrO and graphene oxide–ferric oxide were used to convert glucose into HMF; however, the metal oxide catalysts fully lost their catalytic activities after calcination.14 Zhang et al. also reported the conversion of glucose to HMF over hydroxyapatite supported chromium chloride; however, the yield was below 40%.18 Another study by Jadhav et al. focused on the application of zeolites in ionic liquid for producing HMF from D-glucose; however, the ionic liquids they used were toxic and expensive.19
Therefore, designing a high yield and low cost catalyst is considered to be a highly important aspect of the conversion of glucose to HMF. Reported work by Ronen et al. studied the formation of fructose via isomerization, mainly from Lewis sites, and HMF from both Brønsted and Lewis sites.20 Alam and co-workers reported titanium hydrogen phosphate (TiHP) material consisting of both Brønsted and Lewis acid sites; they proposed that the Brønsted acid sites could accelerate the produce of furan ring opening products when the temperature was greatly increased.21 Therefore, a catalyst with a combination of both Lewis acid sites (for isomerization) and Brønsted acid sites (for the dehydration configuration) may be more promising for the dehydration of glucose to HMF.21–24
New metal phosphates20,25–27 have been proposed for this reaction, in which the metal provides Lewis acid sites and Brønsted acid sites could possibly be obtained from the geminal P(OH) groups.28 A series of metal Zr/Sn(IV) phosphate catalysts with modified surface acid distributions were synthesized; they exhibited improved catalytic performance due to their higher amounts of polyphosphate species.20 Other cerium(IV) phosphate catalysts for this reaction were also reported; however, they showed lower yields (23–24%) and highly stable performance even after several cycles of reaction.27 Much more work still remains to be done to improve the application of cerium phosphate species in the conversion of glucose to HMF.
In the present work, a series of CePO4 catalysts with different crystalline types have been synthesized by a convenient hydrothermal method at different temperatures, and their activity has been studied for the dehydration of glucose to HMF. The relationships of total acid amount and Brønsted and Lewis acid amounts to yield and conversion have also been studied. Meanwhile, various reaction conditions, such as reaction temperature, time, loading of catalyst, amount of reaction medium and reusability, have also been evaluated to determine the optimal reaction conditions for HMF production.
Experimental
Catalyst preparation
The CePO4(CP) catalysts were prepared by a hydrothermal method. In a typical experiment, Ce(NO3)3 (716 mg) and NaH2PO4 (869 mg) with a mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were dissolved in 20 ml diluted water, respectively; these two solutions were mixed, stirred for 1.5 h and then transferred into a Teflon reactor with a stainless steel autoclave. The above autoclave was heated at different temperatures (120, 140, 160, 180 and 200 °C) for 8 h in an oven, then cooled to room temperature. The resulting solids were filtered and washed with diluted water several times, then dried at 100 °C for 12 h. The obtained catalysts were denoted as CP120, CP140, CP160, CP180 and CP200 according to the hydrothermal temperature.
1 were dissolved in 20 ml diluted water, respectively; these two solutions were mixed, stirred for 1.5 h and then transferred into a Teflon reactor with a stainless steel autoclave. The above autoclave was heated at different temperatures (120, 140, 160, 180 and 200 °C) for 8 h in an oven, then cooled to room temperature. The resulting solids were filtered and washed with diluted water several times, then dried at 100 °C for 12 h. The obtained catalysts were denoted as CP120, CP140, CP160, CP180 and CP200 according to the hydrothermal temperature.
Characterization of catalysts
X-ray diffraction (XRD) patterns of the catalysts were evaluated using a D/max-IIIB diffractometer with Cu Kα radiation (λ = 1.5418 Å) combined with a Ni-filter. The morphology of the CP catalysts was investigated by scanning electron microscopy (SEM) (Hitachi S-4800) with an electron gun working at an accelerating voltage of 5 kV. The textural parameters were evaluated by N2 physisorption using a Micromeritics Tristar II analyzer at −196 °C. X-ray photoelectron spectra (XPS) were acquired on a Kratos-AXIS ULTRA DLD with an Al Kα radiation source. Binding energies (BE) were referenced to the C(1s) binding energy of carbon, which was taken to be 284.7 eV.Temperature-programmed desorption of ammonia (NH3-TPD) was carried out in a fully automatic adsorption instrument (XQ TP-5080, China) with a thermal conductibility detector (TCD) according to the following procedure. A fixed catalyst bed with 100 mg sample was enclosed in a quartz tube and fixed with two balls of quartz-wool. Initially, the sample was heated from room temperature to 100 °C with a heating rate of 10 °C min−1 under He flow (30 ml min−1), and then maintained at this temperature for 60 min. The sample was cooled to room temperature, then switched to NH3 flow (30 ml min−1) for 1 h. Finally, the sample was heated to 700 °C with a rate of 10 °C min−1 under He flow (30 ml min−1).
In situ DRIFTS experiments were performed on a Nicolet 6700 spectrometer with an in situ diffuse reflectance pool and a high sensitivity MCT detector cooled by liquid N2. The catalyst was loaded in a Harrick IR cell and heated to 100 °C under N2 flow (40 ml min−1) for 60 min to remove adsorbed impurities, followed by cooling to room temperature. The background spectrum was collected under a N2 atmosphere and was subtracted from the sample spectra. When the adsorption of NH3 on the sample was investigated, the spectra were recorded with an N2 background. The DRIFTS spectra were recorded by accumulating 32 scans with a resolution of 4 cm−1. The total flow rate of the feeding gas was maintained at 40 ml min−1 (20 ml min−1 N2, 20 ml min−1 NH3).
Catalytic activity measurements
The dehydration reaction of glucose was performed with 50 mg catalyst, 50 to 250 mg glucose and 5 to 25 ml dimethylsulfoxide (DMSO) in a 100 ml three-necked flask placed in an oil bath at 130 to 170 °C for 15 to 150 min under N2 atmosphere with stirring. After the reaction, the mixture was cooled and centrifuged. The obtained liquid was analyzed by high performance liquid chromatography (HPLC). The quantitative analysis of glucose was monitored using HPLC (Shimadzu 20A) equipped with a refractive index detector and a Bio-Rad Aminex HPX-87H column. 5-HMF was analyzed using HPLC (DIONEX Ultimate 3000) with an InterSustain C18 column and a 284 nm UV detector.Results and discussion
XRD characterization
Fig. 1 shows the XRD patterns of the CP catalysts synthesized at different hydrothermal temperatures. For CP120, the diffraction peaks at 2θ of 14.3, 20.0, 25.4, 29.1, 31.4, 37.5, 41.9, 48.2, and 53.3° were ascribed to the hexagonal crystalline structure of CePO4 in Fig. 1A, which match well with the standard data of JCPDS 34-1380. However, with increasing synthesis temperature, the intensity of the peaks at 14.3° and 20.0°, indexed to the (100) and (101) crystal planes of CePO4 with a hexagonal structure,29 respectively, gradually decreased; the former peak was diminished in CP160 and the two peaks generally disappeared in CP180 and entirely vanished in CP200. Meanwhile, new peaks at 17.1, 18.9, 21.3, 27.0, 34.4 and 41.1° were observed in CP160 (Fig. 1C), which were attributed to the (101), (011), (111), (200), (202) and (103) planes of monoclinic crystalline phase CePO4 (JCPDS 32-0199), respectively. This crystalline phase fully dominated the CP200 sample.30 This indicates that the crystal phase has been transformed to a completely monoclinic crystal phase for the CP200 sample. It is interesting to note that CP120 exhibited a hexagonal structure and CP200 presented the monoclinic phase, while CP160, CP180 and CP200 demonstrated a mixture of these two crystal phases. This reveals that the change of hydrothermal temperature can lead to transformation into different crystal phases.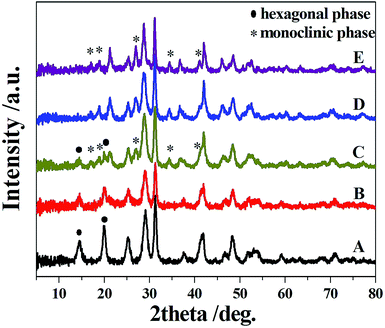 | ||
| Fig. 1 XRD patterns of the CP catalysts ((A) CP120, (B) CP140, (C) CP160, (D) CP180, and (E) CP200). | ||
N2 adsorption–desorption measurements
The textural properties of the CP catalysts derived from nitrogen physisorption are described in Fig. 2a and are summarized in Table 1; the pore distributions are also displayed in Fig. 2b. It can be seen that the CP catalysts exhibited type IV isotherms with a H3 hysteresis loop at 0.4 < p/p0 < 0.9 according to the IUPAC classification,31 which indicates the formation of intercrystal mesopores associated with aggregates of particles in the CP samples.32,33 It is also noteworthy that the pore volumes decreased from 0.39 cm3 g−1 to 0.26 cm3 g−1 (Table 1, Fig. 2). From Fig. 2b, the pore diameter of CP120 was about 25 nm (Fig. 2b-A); the pore diameter widened gradually with increasing hydrothermal temperature, increasing the value more than 46 nm, up to a new pore diameter of 101 nm in CP200. In addition, the BET surface areas were 108, 88, 82, 65 and 54 m2 g−1 for CP120, CP140, CP160, CP180 and CP200, respectively (Table 1), which shows a decrease with increasing synthesis temperature. This results from the change of the crystal phase from hexagonal to monoclinic (Fig. 1). | ||
| Fig. 2 N2 adsorption–desorption isothermals (a) and pore distributions (b) of the CP catalysts ((A) CP120, (B) CP140, (C) CP160, (D) CP180, and (E) CP200). | ||
| Catalyst | SA (m2 g−1) | PV (cm3 g−1) | Total aciditya (mmol g−1) | NH3 adsorptionb (mmol g−1) | Lewis acid amountc (μmol m−2) | Ce4+/(Ce3+ + Ce4+)d (%) | |
|---|---|---|---|---|---|---|---|
| Brønsted (B) | Lewis (L) | ||||||
| a Calculated from NH3-TPD.b Calculated from NH3-TPD and DRIFT based on catalyst mass.c Lewis acid amount based on special surface area.d Calculated from XPS. | |||||||
| CP120 | 108 | 0.39 | 1.67 | 0.74 | 0.93 | 8.6 | 78 |
| CP140 | 88 | 0.39 | 1.39 | 0.70 | 0.69 | 7.8 | 65 |
| CP160 | 82 | 0.36 | 1.29 | 0.82 | 0.47 | 5.7 | 51 |
| CP180 | 65 | 0.30 | 1.21 | 0.90 | 0.31 | 4.8 | 37 |
| CP200 | 54 | 0.26 | 0.79 | 0.51 | 0.28 | 5.2 | 33 |
SEM images
The morphology of the CP catalysts was characterized by SEM, and the results are shown in Fig. 3. It can be easily seen that CP120 displayed moderate aggregation and partial accumulation of a mesosphere structure with particle dimensions of 30 to 60 nm. CP200 denoted obvious nanorod morphology with lengths of 100 to 300 nm and a diameter of 50 nm, while the nanorods showed more cumulated pore structures than CP120. For CP160, a mixed morphology of relatively irregular nanospheres and nanorods was observed. As the synthesis temperature increased, the morphology of the CP catalysts gradually converted from nanospheres (CP120) to nanorods (CP200); the other samples exhibited irregular morphologies due to their mixture of hexagonal and monoclinic crystalline phases.NH3-TPD measurements
The acidity of the CP catalysts was measured by NH3-TPD, and the results are shown in Fig. 4. The CP120 and CP140 catalysts exhibited a series of desorption peaks in the range of 50 to 350 °C, which were attributed to weak NH3 adsorption.23 These peaks demonstrate that there are various surface weak acid sites in CP120 and CP140. It is noteworthy that a small NH3 desorption peak around 450 °C can be observed for CP140, which was assigned to strong acid sites.23 Meanwhile, the desorption peaks around 50 to 350 °C became very weak for CP160, CP180 and CP200; in contrast, the peak at about 500 °C, attributed to the strong acid sites, strengthened. The total amounts of acid sites were calculated by NH3-TPD and were 1.67, 1.39, 1.29, 1.21 and 0.79 mmol g−1 for CP120, CP140, CP160, CP180 and CP200, respectively. This demonstrates that the total acid amount decreases with increasing hydrothermal temperature; CP120 possesses the highest total acid amount, assigned to weak acidity sites, among all the catalysts. These results can be attributed to the full transformation from hexagonal to monoclinic phase. Consequently, this suggests that a CP catalyst with monoclinic crystalline phase may provide strong acid sites, while in another aspect, weak acid is mainly formed in the hexagonal crystalline structure. | ||
| Fig. 4 NH3-TPD profiles of the CP catalysts ((A) CP120, (B) CP140, (C) CP160, (D) CP180, and (E) CP200). | ||
NH3-DRIFT measurement
As we know, Brønsted and Lewis acid sites cannot be distinguished clearly by NH3-TPD measurements. Thus, DRIFT spectra of NH3 absorbed on the surface acid sites of the CP catalysts were acquired in order to determine the amounts of Brønsted and Lewis acid. Fig. 5 shows the IR spectra of the CP catalysts after NH3 adsorption. A strong band at 1690 cm−1 was observed for the CP catalysts, which was assigned to the NH4+ bending vibration on Brønsted acid sites;34,35 additionally, two weaker bands at 1221 and 1578 cm−1 were detected and were attributed to the asymmetric and symmetric bending vibrations of coordinated NH3 on Lewis acid sites.36–38 Combined with the total acid amounts from NH3-TPD (Table 1), the amounts of Brønsted and Lewis acids can be calculated according to the peak area ratio of the Brønsted acid site (1690 cm−1) to the total Lewis acid sites (1221 and 1578 cm−1). The results illustrate that the amounts of Lewis acid are 0.93, 0.69, 0.47, 0.31 and 0.28 mmol g−1 for CP120, CP140, CP160, CP180 and CP200, respectively, which exhibits a declining tendency from CP120 to CP200 with increasing synthesis temperature. This can be ascribed to the hexagonal and monoclinic crystalline structures in CP120 and CP200, respectively. However, the amounts of Brønsted acid sites for these catalysts do not exhibit obvious regularity; there is a decrease in the Brønsted acid amount in the CP140 to CP180 catalysts (0.70 to 0.90 mmol g−1). As is known from the XRD results, CP catalysts with different hydrothermal synthesis temperatures exhibit different crystallite types. CP140, CP160 and CP180 exhibit a mixture of two crystalline structures, which causes instability in the Brønsted acid sites due to different surface properties. Moreover, the IR spectra of CP120 and CP140 and CP160 to CP200 demonstrated different surface acid strengths, which is in accordance with the results from NH3-TPD (Fig. 4). | ||
| Fig. 5 DRIFT spectra of the CP catalysts ((A) CP120, (B) CP140, (C) CP160, (D) CP180, and (E) CP200). | ||
XPS measurements
The CP catalysts were further analyzed by XPS to identify their surface natures, and the results are shown in Fig. 6. The complex spectra of Ce3d were decomposed into eight components by a Gaussian–Lorentz fitting procedure (Fig. 6). The sub-bands labeled v1 and u1 represent the 3d104f1 initial electronic state of Ce3+, and the sub-bands labeled u0, u2, u3, v0, v2 and v3 represent the 3d104f0 state of Ce4+.39,40 The relative abundance of the Ce4+ (%) species of each sample was estimated by considering the deconvolution peak areas of the Ce3d binding energies. As seen in Table 1, the Ce4+ amounts of these catalysts from CP120 to CP200 were 78%, 65%, 51%, 37% and 33%, respectively. As the synthesis temperature increased, the content of Ce4+ gradually decreased from CP120 to CP200. As it has been reported, Ce4+ provides a Lewis acid site,36 which means that a decrease in the Ce4+ amount can lead to a decrease in the amount of Lewis acid sites. Thus, CP120 shows the maximum Lewis acid amount, which also certifies the DRIFT results. Fig. 7 gives the plot of Lewis acid amount (Table 1) versus Ce4+ amount (Table 1) for the CP catalysts. It is interesting that a striking linearity is displayed between the Lewis acid amount and the Ce4+ amount of the CP catalysts (Fig. 7A). It was found that the Lewis acid amount decreased with the BET surface area (Table 1). Moreover, a near linearity relation was observed between the Lewis acid amount and BET surface area for the CP catalysts (Fig. S1†). Considering the effect of the surface area, Lewis acid amounts on the basis of surface area were calculated for the CP catalysts and are shown in Table 1. The plot of surface Ce4+ amount versus Lewis acid amount on the basis of surface area (Table 1) for the CP catalysts is given in Fig. 7B. A linear relation was also found between the surface Ce4+ amount and the Lewis acid amount on the basis of surface area, which suggests that surface area has a positive influence on the Lewis acid amount. Thus, this result proves that Lewis acidity is related to the surface Ce4+ content of the CP catalysts, and its amount is proportional to the content of surface Ce4+. | ||
| Fig. 7 Plots of (A) Lewis acid amount based on catalyst mass (mmol g−1) and (B) Lewis acid amount based on surface area (μmol m−2) versus Ce4+ amount for the CP catalysts. | ||
Catalytic performance
The catalytic performances of the CP catalysts were investigated for the dehydration of glucose to HMF, as displayed in Fig. 8. A lower than 1% conversion of glucose was observed in this reaction without catalyst addition, which indicates the superior stability of glucose under these conditions.41 From Fig. 8, the conversions of glucose were 97%, 90%, 84%, 80% and 78% for CP120, CP140, CP160, CP180 and CP200, respectively; the corresponding yields of HMF for these CP catalysts were 61%, 52%, 45%, 41% and 40%. It can be clearly seen that the yield of HMF and the conversion of glucose decrease with increasing synthesis temperature from CP120 to CP200. Among these CP catalysts, the CP120 catalyst showed the best catalytic performance for the conversion of glucose (97%) and yield of HMF (61%), which is better than most catalysts reported in the literature,12,20,23,42 such as SO42−/ZrO2, SO42−/ZrO2–Al2O3, niobia/carbon, aluminium doped MCM-41 silica and solid metal(IV) phosphate. Jiménez-López and co-workers reported that a glucose conversion of 69% and an HMF yield of 23% were achieved at 175 °C over mesoporous tantalum oxide as the catalyst.12 Dibenedetto et al. used the same elements (Ce, P, O) to prepare different materials with complex structures. These materials were used to catalyze the conversion of fructose to HMF; the best yield of HMF obtained was 52%, and the deactivation phenomenon was also observed.27As we have shown in Fig. 1, the structure of the CP catalysts changed from hexagonal (CP120) to monoclinic (CP200) with increasing synthesis temperature, along with different catalytic performances. This indicates that the crystalline structure has a noticeable influence on the catalytic performance of the CP catalysts, which can be ascribed to the change in surface acidity with the modification of the catalyst structure from hexagonal to monoclinic phase. Generally, the dehydration of glucose to HMF contains two stages: first, isomerization of glucose to fructose at the Lewis acid sites, and then fructose dehydration to HMF over Brønsted acid sites.23,24 In order to study the stages, fructose dehydration to HMF was carried out over CP120 for 5, 10 and 15 min (Fig. S1†). When the reaction time was 5 min, the conversion to fructose and the selectivity for HMF reached about 93% and 49%, respectively. As the time increased to 10 min, the glucose was almost completely converted to fructose (97%), with a corresponding 55% selectivity for HMF. From these results, it can be seen that the stage of fructose dehydration to HMF was very fast. In our previous report, it was also confirmed that the dehydration of fructose to HMF was very fast over carbon-based solid acid catalysts.43 Moreover, when compared with the dehydration of glucose to HMF, the conversion to glucose and the selectivity for HMF with a reaction time of 15 min under the same reaction conditions were only 66% and 40% (Fig. S2†), respectively, which suggests that the reaction rate of glucose dehydration to HMF is slower than that of fructose dehydration to HMF. Therefore, the isomerization of glucose to fructose is considered to be the rate determining step in the dehydration of glucose to HMF. Meanwhile, the isomerization of glucose to fructose is sensitive to Lewis acid in the initial stage of the dehydration of glucose to HMF; therefore, modification of the catalyst acidity will be very important for the reaction.
As was mentioned, the structures of the CP catalysts changed from hexagonal (CP120) to monoclinic (CP200) phase; simultaneously, the crystal morphologies were converted from nanospheres (CP120) to nanorods (CP200) in accordance with the decrease of the BET surface area. The CP catalysts exhibited specific surface Lewis and Brønsted acid sites (Table 1). CP120 had the highest Lewis acid content among all the CP catalysts, as shown in Fig. 5 and Table 1, and it also showed the best activity. From CP140 to CP200, as the Lewis acid amounts gradually decreased, they also displayed decreasing activity for the dehydration of glucose with decreasing amount of Lewis acid, although they had large amounts of Brønsted acid sites. It is interesting that a striking linearity relation can be found between the conversion of glucose and the amount of Lewis acid for the CP catalysts (Fig. 9A). The relationship of selectivity for HMF versus the amount of Lewis acid also demonstrates the same situation as the above described results (Fig. 9A). Considering the effect of surface area, plots of the conversion to glucose and the selectivity for 5-HMF versus Lewis acid amount on the basis of surface area (Table 1) for the CP catalysts are given, respectively (Fig. 9B). A highly linear relationship was also found between the conversion to glucose (selectivity for 5-HMF) and Lewis acid amount on the basis of surface area. These results prove that the surface Lewis acid plays an important role in the dehydration of glucose to HMF.11,14 It also confirms that the isomerization of glucose to fructose is a key stage in this reaction. In addition, the results in Fig. 9B also reveal the positive influence of the surface area on the catalytic activity of the CP catalysts. Thus, the highest conversion (97%) and selectivity (63%) were achieved with CP120, which should be attributed to the highest amount of Lewis acid sites and the highest surface area. However, glucose cannot be completely converted to HMF, which should be ascribed to the secondary processes of HMF rehydration and the condensation of sugars into humins.44,45
More details of CP120 based on its excellent catalytic activity have been investigated in order to understand the effects of various experimental parameters on the reaction, including reaction temperature, time, DMSO amount and ratio of glucose to catalyst.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. It is notable to see that the conversion was almost unchanged with increasing amount of glucose; however, HMF selectivity obviously showed a different tendency. When the ratio of catalyst to glucose decreased from 1
5. It is notable to see that the conversion was almost unchanged with increasing amount of glucose; however, HMF selectivity obviously showed a different tendency. When the ratio of catalyst to glucose decreased from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, the HMF selectivity gradually increased from 41% to 62%; however, when the ratio was 1
4, the HMF selectivity gradually increased from 41% to 62%; however, when the ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, the selectivity of HMF appeared to decrease rapidly to 43%, which in turn also lowered the HMF yield to 41%. When the amount of catalyst is too great, interactions will occur between glucose and newly generated HMF on the catalyst surface, which leads to lower HMF selectivity due to the formation of formic and levulinic acids as well as humins.41 However, a lower amount of catalyst cannot completely catalyze the reaction. From the above result, the conclusion can be made that the optimum ratio of catalyst to glucose in this reaction is 1
5, the selectivity of HMF appeared to decrease rapidly to 43%, which in turn also lowered the HMF yield to 41%. When the amount of catalyst is too great, interactions will occur between glucose and newly generated HMF on the catalyst surface, which leads to lower HMF selectivity due to the formation of formic and levulinic acids as well as humins.41 However, a lower amount of catalyst cannot completely catalyze the reaction. From the above result, the conclusion can be made that the optimum ratio of catalyst to glucose in this reaction is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.
4.
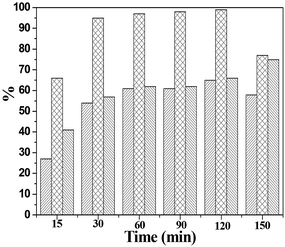
The results presented in Fig. 10–13 suggest that CP120 catalyst shows an excellent catalytic performance for glucose dehydration to 5-(hydroxymethyl)furfural under the conditions of 50 mg catalyst, 200 mg glucose, 160 °C, 60 min and 10 ml DMSO as solvent.
Conclusions
A series of CePO4 catalysts with hexagonal and/or monoclinic CePO4 crystal types have been successfully synthesized by a hydrothermal method. The increase in the hydrothermal temperature caused a gradual change in the catalyst crystal phase from hexagonal to monoclinic, which led to changes in the BET surface area and acidity. The catalytic performance combined with the characterization results suggests that the surface acid strength and the distribution of different types of acid sites influence the catalyst activity significantly. The amount of Lewis acid based on both catalyst mass and surface area increases with the surface Ce4+ content for these CP catalysts, showing a striking linearity. A very good linearity is also displayed between Lewis acid amount based on either catalyst mass or surface area and conversion of glucose and selectivity of HMF. Therefore, we propose that Lewis acidity derived from the surface Ce4+ of the CP catalysts plays a key role in the dehydration of glucose to HMF. The best catalytic performance is obtained over CP120 nanoparticles at 30 to 60 nm in the dehydration of glucose to HMF, which exhibits a HMF yield of 61% with a glucose conversion of 97% using 10 mL DMSO as solvent, 50 mg catalyst and 200 mg glucose at 160 °C for 60 min. This is attributed to the fact that CP120 has the highest amount of Lewis acid and the highest BET surface area among these CP catalysts.Acknowledgements
This study was supported by the Natural Sciences Fund of Heilongjiang Province (B2015009), Postdoctoral Science-research Developmental Foundation of Heilongjiang Province of China (LBH-Q12022), Program for Innovative Research Team in University (IRT-1237), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (2013-1792) and Ministry of Human Resources and Social Security (2013-277), Innovative Research Project of Key Laboratory of Functional Inorganic Material Chemistry, Ministry of Education.References
- B. Saha and M. M. Abu-Omar, Green Chem., 2014, 16, 24–38 RSC.
- S. P. Teong, G. Yi and Y. Zhang, Green Chem., 2014, 16, 2015–2026 RSC.
- E. R. Sacia, M. Balakrishnan and A. T. Bell, J. Catal., 2014, 313, 70–79 CrossRef CAS.
- L. Hu, G. Zhao, W. Hao, X. Tang, Y. Sun, L. Lin and S. Liu, RSC Adv., 2012, 2, 11184–11206 RSC.
- T. Wang, M. W. Nolte and B. H. Shanks, Green Chem., 2014, 16, 548–572 RSC.
- W. Deng, Q. Zhang and Y. Wang, Sci. China: Chem., 2014, 58, 29–46 CrossRef.
- H. Cai, C. Li, A. Wang, G. Xu and T. Zhang, Appl. Catal., B, 2012, 123–124, 333–338 CrossRef CAS.
- X. Tong, Y. Ma and Y. Li, Appl. Catal., A, 2010, 385, 1–13 CrossRef CAS.
- M. J. Climent, A. Corma and S. Iborra, Green Chem., 2011, 13, 520 RSC.
- J. Zhou, Z. Xia, T. Huang, P. Yan, W. Xu, Z. Xu, J. Wang and Z. C. Zhang, Green Chem., 2015, 17, 4206–4216 RSC.
- A. Osatiashtiani, A. F. Lee, D. R. Brown, J. A. Melero, G. Morales and K. Wilson, Catal. Sci. Technol., 2014, 4, 333–342 CAS.
- I. Jiménez-Morales, M. Moreno-Recio, J. Santamaría-González, P. Maireles-Torres and A. Jiménez-López, Appl. Catal., B, 2014, 154–155, 190–196 CrossRef.
- X. Qi, M. Watanabe, T. M. Aida and R. L. Smith Jr, Catal. Commun., 2009, 10, 1771–1775 CrossRef CAS.
- M. Zhang, K. Su, H. Song, Z. Li and B. Cheng, Catal. Commun., 2015, 69, 76–80 CrossRef CAS.
- V. V. Ordomsky, J. V. D. Schaaf, J. C. Schouten and T. A. Nijhuis, J. Catal., 2012, 287, 68–75 CrossRef CAS.
- M. Dashtban, A. Gilbert and P. Fatehi, RSC Adv., 2014, 4, 2037–2050 RSC.
- Y. Yang, C. Hu and M. M. Abu-Omar, J. Mol. Catal. A: Chem., 2013, 376, 98–102 CrossRef CAS.
- Z. Zhang and Z. K. Zhao, Bioresour. Technol., 2011, 102, 3970–3972 CrossRef CAS PubMed.
- H. Jadhav, E. Taarning, C. M. Pedersen and M. Bols, Tetrahedron Lett., 2012, 53, 983–985 CrossRef CAS.
- R. Weingarten, Y. T. Kim, G. A. Tompsett, A. Fernández, K. S. Han, E. W. Hagaman, W. C. Conner Jr, J. A. Dumesic and G. W. Huber, J. Catal., 2013, 304, 123–134 CrossRef CAS.
- M. I. Alam, S. De, B. Singh, B. Saha and M. M. Abu-Omar, Appl. Catal., A, 2014, 486, 42–48 CrossRef CAS.
- R. Gounder and M. E. Davis, J. Catal., 2013, 308, 176–188 CrossRef CAS.
- H. Yan, Y. Yang, D. Tong, X. Xiang and C. Hu, Catal. Commun., 2009, 10, 1558–1563 CrossRef CAS.
- T. D. Swift, H. Nguyen, Z. Erdman, J. S. Kruger, V. Nikolakis and D. G. Vlachos, J. Catal., 2016, 333, 149–161 CrossRef CAS.
- S. Xu, X. Yan, Q. Bu and H. Xia, RSC Adv., 2016, 6, 8048–8052 RSC.
- L. Yang, X. Yan, S. Xu, H. Chen, H. Xia and S. Zuo, RSC Adv., 2015, 5, 19900–19906 RSC.
- A. Dibenedetto, M. Aresta, C. Pastore, L. di Bitonto, A. Angelini and E. Quaranta, RSC Adv., 2015, 5, 26941–26948 RSC.
- A. Sinhamahapatra, N. Sutradhar, B. Roy, A. Tarafdar, H. C. Bajaj and A. B. Panda, Appl. Catal., A, 2010, 385, 22–30 CrossRef CAS.
- J. Bao, R. Yu, J. Zhang, X. Yang, D. Wang, J. Deng, J. Chen and X. Xing, CrystEngComm, 2009, 11, 1630–1634 RSC.
- K. Rajesh, P. Mukundan, P. K. Pillai, V. R. Nair and K. G. K. Warrier, Chem. Mater., 2004, 16, 2700–2705 CrossRef CAS.
- R. Amrousse, A. Tsutsumi, A. Bachar and D. Lahcene, Appl. Catal., A, 2013, 450, 253–260 CrossRef CAS.
- L. Ho, H. Nishiguchi, K. Nagaoka and Y. Takita, Mater. Chem. Phys., 2006, 97, 494–500 CrossRef CAS.
- M. Choi, R. Srivastava and R. Ryoo, Chem. Commun., 2006, 42, 4380–4382 RSC.
- M. A. Larrubia, G. Ramis and G. Busca, Appl. Catal., B, 2000, 27, L145–L151 CrossRef CAS.
- A. Trovarelli, Catal. Rev., 2006, 38, 439–520 Search PubMed.
- L. Chen, J. Li and A. M. Ge, Environ. Sci. Technol., 2010, 44, 9590–9596 CrossRef CAS PubMed.
- Z. Wu, B. Jiang, Y. Liu, H. Wang and A. R. Jin, Environ. Sci. Technol., 2007, 41, 5812–5817 CrossRef CAS PubMed.
- W. S. Kijlstra, D. S. Brands, E. K. Poels and A. Bliek, J. Catal., 1997, 171, 208–218 CrossRef CAS.
- M. Piumetti, S. Bensaid, N. Russo and D. Fino, Appl. Catal., B, 2015, 165, 742–751 CrossRef CAS.
- P. Maitarad, J. Han, D. Zhang, L. Shi, S. Namuangruk and T. Rungrotmongkol, J. Phys. Chem. C, 2014, 118, 9612–9620 CAS.
- V. V. Ordomsky, V. L. Sushkevich, J. C. Schouten, J. van der Schaaf and T. A. Nijhuis, J. Catal., 2013, 300, 37–46 CrossRef CAS.
- H. Xiong, T. Wang, B. H. Shanks and A. K. Datye, Catal. Lett., 2013, 143, 509–516 CrossRef CAS.
- C. Wang, F. Yuan, L. Liu, X. Niu and Y. Zhu, ChemPlusChem, 2015, 80, 1657–1665 CrossRef CAS.
- V. V. Ordomsky, J. van der Schaaf, J. C. Schouten and T. A. Nijhuis, ChemSusChem, 2012, 5, 1812–1819 CrossRef CAS PubMed.
- B. F. M. Kuster, Carbohydr. Res., 1977, 54, 177–183 CrossRef CAS.
- Y. Wang, Z. Gu, W. Liu, Y. Yao, H. Wang, X. Xia and W. Li, RSC Adv., 2015, 5, 60736–60744 RSC.
- C. B. Rasrendra, J. N. M. Soetedjo, I. G. B. N. Makertihartha, S. Adisasmito and H. J. Heeres, Top. Catal., 2012, 55, 543–549 CrossRef CAS.
- R. M. Musau and R. M. Munavu, Biomass, 1987, 13, 67 CrossRef CAS.
- Y. Yang, W. Liu, N. Wang, H. Wang, Z. Song and W. Li, RSC Adv., 2015, 5, 27805–27813 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra02602h |
| This journal is © The Royal Society of Chemistry 2016 |