Response of sludge fermentation liquid and microbial community to nano zero-valent iron exposure in a mesophilic anaerobic digestion system†
Bao Yu,
Xiaoting Huang,
Dongling Zhang,
Ziyang Lou,
Haiping Yuan and
Nanwen Zhu*
School of Environmental Science and Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240, P. R. China. E-mail: nwzhu@sjtu.edu.cn; Fax: +86 021 54743710; Tel: +86 021 54743710
First published on 26th February 2016
Abstract
The effects of nano zero-valent iron (NZVI) on sludge anaerobic digestion were investigated from the perspective of the sludge fermentation liquor (SFL) and microbial community structure. Compared with micrometer-scale ZVI, NZVI exhibited a considerable inhibition in methane production during the initial 6 days while the negative effect attenuated subsequently and methane production recovered. Similar to micrometer-scale ZVI, enhanced methane production (108.24 mL per gVS) was obtained with NZVI addition, and increased by 46.1% compared to no-ZVI assay. The results indicated that NZVI could promote hydrolysis-acidification with inhibited conversion of acetic acid to methane in the initial stage, which could be ascribed to the high H2 partial pressure. The rapid dissolution of NZVI hindered the phosphorus uptake by methanogens, meanwhile the more reductive atmosphere contributed to the degradation of propionic acid. Further investigations on SFL showed that NZVI could facilitate the release of biodegradable compounds without propionic acid accumulation, creating a favorable substrate environment for methanogens. The bacteria and archaea community structure involved in methane production was studied. Results indicated that NZVI could enhance hydrogenotrophic methanogenesis with the highest relative abundances of Clostridia (53.2%) and Methanosarcina (22.6%) among the assays.
1. Introduction
With the increasing promotion and application of nanotechnology, large numbers of nanomaterials are used in consumer and industrial products such as semiconductors, personal care products, pigments and textiles.1 Meanwhile, the application of engineered nanomaterials (ENMs) in environmental remediation has increased continuously in recent decades,2 owing to their larger available surface area and superior reactivity.3 The applications of nanomaterials in commercial products are expected to gradually improve the quality of the environment and improve our lives. However, high reactivity correlates with low selectivity, which tends to react with non-target substances, and poses potential toxic effect on the health of humans and components of the ecosystems.4 Thereby, it is imperative to understand the environmental impact of nanomaterials.Nano zero-valent iron (NZVI) is certainly one of the most widely applied nanomaterials, especially as an environmental remediation reagent for groundwater, soil and hazardous waste treatment.5 Numerous challenging contaminants in the environment could be degraded or adsorbed by NZVI, for instance dechlorination of halogenated hydrocarbons, reduction of nitroaromatic compounds, degradation of azo dyes, and adsorption of heavy metal ions.6 The wide utilization of NZVI has inevitably caused their release into the environment, such as into the soil, natural water bodies and wastewater treatment plants (WWTPs).7 As the last barrier prior to environmental release, WWTPs are important for blocking NZVI from entering the natural environment, and NZVI would eventually end up in sludge by adsorption.8 Large amounts of waste activated sludge (WAS) produced in WWTPs need to be treated before discharging to the environment. Anaerobic digestion (AD) is considered as an interesting option for WAS treatment since both pollution control and energy recovery (methane) could be achieved.9
It is demonstrated that ZVI powder could lower oxidation-reduction potential (ORP) and create a more favorable environment for anaerobic biological processes.10 While some reports have suggested that the presence of NZVI in aqueous solution (e.g. wastewater) adversely affects the microorganisms. For instance, Bacillus subtilis and Pseudomonas fluorescens could be inactivated when the dosage of NZVI exceeded 10 mg mL−1;11 Xiu et al. reported that addition of NZVI could pose deleterious effect on microbial reduction of trichloroethylene (TCE) and inhibit dechlorinating organisms.12 The impact of NZVI on soil microbial structure was also investigated and it was dependent on dosage and microbial species.13 A variety of toxicity mechanisms have been proposed, including disruption of the cell membrane integrity, damage of enzymatic proteins and interference with respiration.14 The function of ZVI primarily seemed to depend on iron corrosion kinetics and eventual electron transfer in aqueous media. The surface reaction consists of two reaction steps: (1) hydrogen ion adsorbed on the iron surface and (2) reduced to hydrogen atoms, subsequently forming hydrogen gas.15 Obviously, the high reactivity of NZVI is linked to its large surface, which might account for the inhibitory effect on microorganisms.
Although it is known that NZVI could affect biological systems, most studies so far focused on the potential influences on soil microbial populations or microorganisms in pure culture, the response relationship between NZVI and microbial community structure in sludge AD system was not established to date. Furthermore, the effects of NZVI towards sludge fermentation liquor (SFL) remain unclear. SFL plays an important role in AD system, providing liquor phase environment, carbon source and acid/base buffering capacity.16 The changes of SFL will directly affect the overall performance of sludge AD process. Therefore, the main objective of this study was to identify how NZVI and its bulk counterpart (ZVI powder at micrometer level) affect sludge AD system from the perspective of SFL and microbial community structure. The biogas (methane and carbon dioxide) production performance was compared firstly. Aside from measuring the composition of the volatile fatty acid (VFA) during hydrolysis-acidification and full-scale fermentation processes, the dissolved organic matter (DOM) species and contents of SFL were examined using excitation–emission matrix (EEM) for microbial metabolism analysis. In addition, the microbial community structures were also investigated to reveal the reason for the AD performance under the test conditions by 454 high-throughput pyrosequencing.
2. Experimental
2.1 Characteristics of WAS and NZVI
WAS was collected from a municipal wastewater treatment plant in Shanghai, China. Before further use, the sludge was sieved to size of less than 1.0 mm, and then thickened to total solid (TS) of ∼3.5%. The pretreated sludge samples were stored in the refrigerator at 4 °C. Anaerobic digested sludge used as inoculum was collected from a mesophilic digester and the main characteristics are shown in Table 1.| Parameters | WAS | Seed sludge |
|---|---|---|
| a TS: total solid; VS: volatile solid; TCOD: total chemical oxygen demand; SCOD: soluble chemical oxygen demand. | ||
| pH | 6.92–7.10 | 6.90–7.04 |
| TS (mg L−1) | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740–32 740–32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 950 |
62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400–63 400–63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
| VS (mg L−1) | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500–24 500–24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200–44 200–44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
| TCOD (mg L−1) | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600–35 600–35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700–44 700–44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
| SCOD (mg L−1) | 160–192 | 1020–1150 |
| Soluble proteins (mg L−1) | 10.8–11.2 | 63.2–76.6 |
| Soluble carbohydrates (mg L−1) | 19.2–20.4 | 87.2–98.4 |
NZVI particles were purchased from Aladdin Industrial Inc., Shanghai, China. The NZVI particles were observed by high resolution transmission electron microscopy (HR-TEM) performed with a JEM-2100 operated at 200 kV (JEOL, Tokyo, Japan) as shown in Fig. S1.† The size of NZVI particles was in the range of 5–100 nm with the BET specific surface area of 25 m2 g−1 (purity > 99.9%). Highly concentrated stock slurry of 100 g L−1 NZVI was prepared in an anoxic chamber, which was dispersed by ultrasonic treatment. Further experiments were then carried out by preparing dilutions from the stock slurry as required.
2.2 Batch experiments
The batch experiments were conducted in identical reactors with the mixture of inoculum and raw sludge at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (based on total solids). All the reactors incubated at mesophilic digestion temperature of 35 ± 2 °C controlled by the water-bath shaker (100 rpm). To investigate the effects of NZVI on hydrolysis-acidification and methanogenesis respectively, the experiments were divided into two stages. The first experiment lasted for 3 days to explore the effect of NZVI on hydrolysis-acidification, and the other experiment (full-scale) was conducted for 32 days to investigate the effect on the whole AD process including hydrolysis-acidification and methanogenesis. All anaerobic fermentation tests were operated in triplicate and one way analysis of variance (ANOVA) at 0.05 level was used to analyze the data.
9 (based on total solids). All the reactors incubated at mesophilic digestion temperature of 35 ± 2 °C controlled by the water-bath shaker (100 rpm). To investigate the effects of NZVI on hydrolysis-acidification and methanogenesis respectively, the experiments were divided into two stages. The first experiment lasted for 3 days to explore the effect of NZVI on hydrolysis-acidification, and the other experiment (full-scale) was conducted for 32 days to investigate the effect on the whole AD process including hydrolysis-acidification and methanogenesis. All anaerobic fermentation tests were operated in triplicate and one way analysis of variance (ANOVA) at 0.05 level was used to analyze the data.
In the first experiment, 200 mL of the mixture sludge was added into four serum bottles with the working volume of 250 mL. Afterwards, certain dosage of micrometer-scale ZVI (100 mesh and 1000 mesh respectively, purity > 98%) and NZVI were added to three bottles at the final concentration of 10 g L−1, which was based on the successful use of ZVI powder for the enhanced methane production.17 The control assay was also carried out under the same operation conditions without ZVI. To get rid of methanogens from anaerobic fermentation system, 40 mM 2-bromoethanesulfonate (Sigma-Aldrich Corporation, USA) was added as methanogenic inhibitor to prevent the consumption of VFAs.18 After removing oxygen from the headspace, each bottle was capped with rubber stoppers. Another experiment was operated in Erlenmeyer flasks with the working volume of 2 L. The operation conditions were same as the hydrolysis-acidification experiment, except that no 2-bromoethanesulfonate was added. The digestion lasted for 32 days by which stage the methane production nearly ceased, and the pH value was not adjusted during the entire process.
2.3 Analytical methods
Sludge samples were collected to measure pH, ORP, total solids (TS), volatile solids (VS) and total chemical oxygen demand (TCOD). The sludge supernatant was collected by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min and filtrating through 0.45 μm cellulose membrane. Soluble chemical demand (SCOD), total nitrogen (TN) and total phosphate (TP) were measured in accordance with the standard methods.19 Soluble protein was measured by Lowry's method and soluble carbohydrates were determined by the phenol-sulfuric method.20,21 A gas chromatograph (GC-7890, Agilent) was used to determine VFAs, namely acetic, propionic, iso-butyric, n-butyric, iso-valeric and n-valeric acids. The composition of biogas were quantified by a gas chromatograph (GC-14B, Shimadzu) and the method was followed the description in our previous study.22 The conversion coefficients of protein, carbohydrates, acetate, propionate, butyrate and valerate to COD were 1.5, 1.06, 1.07, 1.51, 1.82 and 2.04, respectively. Microwave digestion system (ETHOS ONE, MILESTONE) was applied to pretreat the sludge solids with a mixture of HNO3/H2O2/HF as digestion solvents, and then the concentrations of iron ion in the sludge solids and supernatant were determined by Inductively Coupled Plasma Optical Emission Spectrometer (ICP-PS3500DD, HITACHI). The elements C, H, N and S in sludge were measured with the elemental analyzer (Vario EL Cube, Elementar).
000 rpm for 20 min and filtrating through 0.45 μm cellulose membrane. Soluble chemical demand (SCOD), total nitrogen (TN) and total phosphate (TP) were measured in accordance with the standard methods.19 Soluble protein was measured by Lowry's method and soluble carbohydrates were determined by the phenol-sulfuric method.20,21 A gas chromatograph (GC-7890, Agilent) was used to determine VFAs, namely acetic, propionic, iso-butyric, n-butyric, iso-valeric and n-valeric acids. The composition of biogas were quantified by a gas chromatograph (GC-14B, Shimadzu) and the method was followed the description in our previous study.22 The conversion coefficients of protein, carbohydrates, acetate, propionate, butyrate and valerate to COD were 1.5, 1.06, 1.07, 1.51, 1.82 and 2.04, respectively. Microwave digestion system (ETHOS ONE, MILESTONE) was applied to pretreat the sludge solids with a mixture of HNO3/H2O2/HF as digestion solvents, and then the concentrations of iron ion in the sludge solids and supernatant were determined by Inductively Coupled Plasma Optical Emission Spectrometer (ICP-PS3500DD, HITACHI). The elements C, H, N and S in sludge were measured with the elemental analyzer (Vario EL Cube, Elementar).
2.4 EEM fluorescence spectra and FRI analysis
The EEM fluorescence spectra of DOM were obtained by Hitachi FL-7000 (Japan) fluorescence spectrophotometer at a room temperature (approximately 25 °C). The scanning speed, photomultiplier tube voltage and slit width were set to 2400 nm min−1, 600 mV and 5 nm, respectively. The excitation wavelength (Ex) increased from 200 to 500 nm at 10 nm increments, while the emission wavelength (Em) varied between 250 and 600 nm at 10 nm intervals. Raleigh scattering was subtracted with the DI water as the blank.23Fluorescence regional integration (FRI) technique was applied to quantitatively determine the five organic matters (namely tyrosine, tryptophan, fulvic acid-like, soluble microbial by-product-like and humic acid-like substances) in the regions (Region I, II, III, IV and V) of EEM fluorescence.23 The normalized Ex/Em area volumes (Øi,n, ØT,n) and percent fluorescence response (Pi,n, %) were calculated as eqn (1)–(3):
 | (1) |
| ØT,n = ∑Øi,n | (2) |
| Pi,n = Øi,n/ØT,n × 100% | (3) |
In which Øi is the volume beneath region “i” of the EEM. Δλex and Δλem are the excitation wavelength interval (taken as 10 nm) and emission wavelength interval (taken as 10 nm). I(λexλem) denotes the fluorescence intensity (au) at each excitation–emission wavelength pair. MFi is equal to the inverse of the fractional projected excitation–emission area.
2.5 DNA extraction and high throughput pyrosequencing
5 mL fermentation broth was collected from the bottom of reactors at the end of mesophilic anaerobic digestion, and then stored in freezing tubes at −80 °C for further microbial community analysis. FastDNA® Spin Kit for Soil was applied to extract DNA according to the manufacturer's instructions. DNA integrity was tested by agarose gel electrophoresis (AGE) and DNA quality was checked on a 0.8% agarose gel. The primers for bacteria were 8F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 533R (5′-TTACCGCGGCTGCTGGCAC-3′), whereas the primers designed for archaea were Arch787F (5′-ATTAGATACCCSBGTAGTCC-3′) and Arch1059R (5′-GCCATGCACCWCCTCT-3′). The thermal program for amplification of 16rRNA gene was according to our previous study.22 The PCR products were purified and quantified, then pooled at equal concentrations; then the PCR products of 16S rRNA gene were sequenced on Roche 454 platform (Branford, CT, USA) according to the standard protocols with the paired-end (PE) configuration of 2 × 250. The raw fastq files were demultiplexed by Qiime platform and effective sequences was clustered into operation taxonomic unit (OTU) with 97% similarity cutoff. The phylogenetic affiliation of 16S rRNA gene sequence was analyzed using RDP Classifier against Greengenes 16S rRNA gene database.243. Results and discussion
3.1 Performance of biogas production
Biogas, as a by-product of AD process, is mainly composed of methane and carbon dioxide, and the cumulative methane production is an important index to assess the performance of AD.17 The cumulative CH4 and CO2 production vs. fermentation time are recorded in Fig. 1. After 32 days' digestion, the cumulative CH4 production was 171.62, 142.69 and 108.24 mL per gVS in the assays with 100 mesh ZVI (ZVI-100), 1000 mesh ZVI (ZVI-1000) and nano ZVI (NZVI), which increased by 131.6%, 92.6% and 46.1% than the no-ZVI dosage assay (Control), respectively. The results indicated that ZVI (both micrometer-scale and nano ZVI) had favorable effects on the AD performance. Particularly, NZVI had a significant inhibitory effect on methane production during the initial stage. For example, of the total bio-methane produced in the full-scale fermentation process, only 13.5% was produced in about 3 days' digestion, while that in the control assay was 32.9%; thereafter the methanogenic activity recovered and the methane production increased gradually, which was in agreement with the observations during the dechlorinating trichloroethylene process.12 In terms of the cumulative CO2 production, the following order: ZVI-100 (51.83 mL per gVS) > ZVI-1000 (39.18 mL per gVS) > Control (17.48 mL per gVS) > NZVI (4.08 mL per gVS) was observed. Higher cumulative CO2 production in the assays with ZVI-100 and ZVI-1000 indicated more substrates were mineralized, which was consistent with the methane production. In contrast, the sludge treated with NZVI showed no significant CO2 production, which was beneficial to the quality of biogas.It has been demonstrated that ZVI could enhance the activity of methanogens, contributing to the enhancement of methane production.25 ZVI could also directly serve as an electron donor for reducing CO2 into CH4 through hydrogenotrophic methanogenesis via eqn (4), and transfer electron to microorganisms via the microbial corrosion and surface oxidation.26 In eqn (5), ZVI is oxidized to ferrous iron with the production of H2. Besides, H2 as a by-product was also produced during the hydrolysis-acidification process, while very little H2 was detected when sludge treated with ZVI-100 and ZVI-1000 (Fig. S2†). The consumption of H2 could be ascribed to the effective utilization by hydrogenotrophic methanogens via eqn (6). The above results demonstrated the positive effects of ZVI on methane production. Compared to that of ZVI, the faster dissolution of NZVI not only resulted in more H2 production and accumulation, but also led to the alkali pH. As shown in Fig. S3,† the pH of all the reactors increased with the final values of 7.32 (Control), 7.69 (ZVI-100), 8.28 (ZVI-1000) and 9.03 (NZVI), respectively. The less production of CO2 might be also ascribed to the alkaline pH since the evolution of CO2 generation was blocked. Furthermore, the fast H2 generation from NZVI dissolution might be involved in methanogenesis inhibition in the first three days with the H2 partial pressure of 21 Pa (Fig. S2†). As reported previously, the high H2 partial pressure was detrimental to methane production because of the associated CO production and the impeded thermodynamical conversions of propionate and butyrate into acetate.27 Nevertheless, NZVI could also enhance the methane production from AD of WAS in spite of the presence of inhibitory effect in the initial stage.
| CO2 + 4Fe0 + 8H+ → CH4 + 4Fe2+ + 2H2O, ΔG0′ = −150.5 kJ mol−1 | (4) |
| Fe0 + 2H2O → Fe2+ + H2 + 2OH−, ΔG0′ = −5.02 kJ mol−1 | (5) |
| 4H2 + CO2 → CH4 + 2H2O, ΔG0′ = −131 kJ mol | (6) |
3.2 VFAs production and composition
The during the AD process, once the insoluble organics are solubilized and hydrolyzed, they will be converted into long chain fatty acids (LCFAs), sugars and amino acids, and further converted into smaller VFAs.25 As the important components of SFL, VFAs are considered as the key parameters which could significantly influence the performance of AD. Fig. 2 illustrates that the total VFAs level (mainly including acetic, propionic and butyric acids) were only 32.1 mg COD/L at the beginning of test with acetic and propionic acids being the dominant components. As expected, the assay with NZVI achieved a rapid increase and moved up to the peak (610.8 mg COD/L) on 3rd day, indicating part of cell lysis and the release of cellular components due to the disruption of cell membranes by NZVI.27 Afterwards, efficient conversion of VFAs (particularly acetic acid) into methane was observed, and the residual total VFAs concentration was 64.9 mg COD/L after 32 days' digestion. The assay with ZVI-100 also obtained the peaks (294.8 mg COD/L) on 3rd day, and the degradation of VFAs was more effective and complete. Moreover, as compared to the ZVI-100 treatment and the control, the assay with ZVI-1000 had a different trend, that is, total VFAs concentration first decreased and then increased during day 3 to day 32 with the final value of 81.6 COD/L. Interestingly, even though acetic acid in the control assay was degraded rapidly, the accumulation of propionic acid was observed ranging from 37.7 mg COD/L to 215.4 mg COD/L during the full-scale fermentation process. The reason should be attributed to the unfavorable conversion of propionic acid in thermodynamics (ΔG0 = +76.1 kJ mol−1) with the strict requirements in terms of ORP (<−278 mV).26 The results suggested that ZVI (both micrometer-scale and nano ZVI) could enhance the degradation of propionic acid by creating a more reductive atmosphere, and the decrease of propionic acid in AD system could also contribute to the favorable substrate environment for methanogenesis.To investigate the effects of NZVI on the hydrolysis-acidification of WAS, 2-bromoethanesulfonate was added to completely inhibit methanogenesis, and the fermentation lasted for 3 days. The compositions of VFAs in the processes of full-scale fermentation and fermentation without methanogenesis were detected and illustrated in Fig. 3. In comparison with the control assay, ZVI-100, ZVI-1000 and NZVI could enhance hydrolysis-acidification process with the enhanced total VFAs concentrations of 788.7 mg COD/L, 854.3 mg COD/L and 1082.1 mg COD/L, respectively.17 Except for the assay with NZVI, iso-butyric, valeric and iso-valeric acids in other assays were decomposed effectively, which was consistent with the methane production in the initial stage. Furthermore, the reduction of total VFAs in the assays with ZVI-100 and ZVI-1000 reached 490 mg COD/L and 506 mg COD/L respectively, while that in control assay was only 238.9 mg COD/L, indicating ZVI could enhance both hydrolysis-acidification and methanogenesis. In contrast, NZVI could only enhance hydrolysis-acidification with the inhibited conversion of organic acids to methane in the initial stage. The results should be attributed to the higher H2 partial pressure (21 Pa on 3rd day) caused by the rapid dissolution of NZVI, which inhibited acetoclastic methanogenesis.27 Meanwhile, as the energy source, H2 could be used not only by hydrogenotrophic methanogenesis to form extra CH4 with the consumption of part of CO2 but also by homoacetogenic bacteria for the formation of methanogenic precursor acetic acid (eqn (7)).28 The accumulation of acetic acid (217.7 mg COD/L) on 3rd day and less detected CO2 production supported the evidence, and this part of acetic acid also contributed to the enhanced methane production during the full-scale fermentation. The results suggested that NZVI was mainly acting by hindering the utilization of VFAs by acetoclastic methanogens instead of the conversion of CO2/H2 into VFAs through homoacetogenic bacteria in the initial stage.
| 4H2 + 2CO2 → CH3COO− + H+ + 2H2O, ΔG0′ = −95 kJ mol−1 | (7) |
 | ||
| Fig. 3 Comparison of individual VFA between the full-scale fermentation process and fermentation without methanogenesis process on 3rd day. | ||
3.3 Composition of SFL and microbial metabolism analysis
Converting sludge particles to SFL is the first step and those soluble organic matters would be finally utilized by anaerobic microbes, accompanied with the sludge reduction.22 To determine the mechanisms of NZVI on substrate (such as protein, carbohydrate, acetate, propionate, butyrate, amino acid, etc.) degradation during the AD process, the changes of soluble organic compounds in SFL were tracked on 0th (initial stage), 3rd day (highest VFAs accumulation), 17th day (intermediate stage) and 32nd day (terminal stage). As shown in Fig. 4, in the initial stage, the unknown organic matters (such as ethanol, amino acids and LCFA etc.) were the main components in SCOD (264 mg L−1), which contributed to about 68%; while that of acetate, propionate, carbohydrates and proteins were 8%, 6%, 9% and 8%, respectively. After 3 days' digestion, the soluble organic compounds in all the assays increased with SCOD values of 1204 mg L−1 (Control), 1480 mg L−1 (ZVI-100), 1260 mg L−1 (ZVI-1000) and 4080 mg L−1 (NZVI) respectively, indicating the rapid hydrolysis-acidification of WAS. The contribution proportion of unknown organic matters in the assay with NZVI increased to 72%, accompanied with the proportion decrease of acetate (6%). Notably, the supplementation of NZVI resulted in the accumulation of acetate with the highest concentration of 233 mg COD/L, which was in accordance with the conversion of CO2/H2. With the conversion of soluble organic matters into CH4, the concentrations of soluble organic compounds decreased to 580 mg L−1 (Control), 700 mg L−1 (ZVI-100), 740 mg L−1 (ZVI-1000) and 2880 mg L−1 (NZVI) respectively on 17th day, while the proportion of acetate in the assay with NZVI remained at a high level (216 mg COD/L). At the end of digestion, the soluble organic compounds in all assays had been removed at different levels, and the main components in SFL were also unknown organic matters with the proportion of 75% (Control), 84% (ZVI-100), 82% (ZVI-1000) and 79% (NZVI) respectively. In addition, propionate in the assays with micrometer-scale and nano ZVI contributed to less proportion in SFL compared with the control assay, which confirmed the positive effect of ZVI on degradation of propionate.25As the important part of SFL, DOM could be metabolized as direct carbon source, and the compositional and structural characteristics of DOM are particularly useful for studying transformation and fate of soluble organic compounds in the following AD process.29 EEM fluorescence spectroscopy was applied to clarify the bioavailability of fermentation products, especially unknown organic matters in SFL.30 The distribution of biodegradable (tyrosine-like and soluble microbial by-product-like) and non-biodegradable (tryptophan-like, fulvic acid-like, and humic acid-like) compounds in DOM was characterized, and the changes of five Ex/Em regions during the AD process were assessed quantitatively by FRI technique.23 As shown in Fig. 5, Region IV presented in the highest contribution proportion of percent fluorescence response (Pi,n) of 77.40% in the initial stage, followed by region II (16.36%) and region III (6.24%). The results indicated that soluble microbial by-products were the main components with a good biodegradability. After 3 days' digestion, the Pi,n value of Region IV in the assay with NZVI increased to 91.86% with the highest fluorescence intensity of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 056 au (Fig. S4†). The results implied that NZVI could promote the release of biodegradable compounds (including some unknown organic matters) to SFL, which was in agreement with the accumulation of acetate. Meanwhile, the Pi,n values of Region IV in the assays with ZVI-100 (76.46%) and ZVI-1000 (77.95%) were slightly lower than the control assay (80.67%), which might be due to the elevated methanogenesis (P < 0.05). In the intermediate stage, Region II and Region IV still contributed to the main fluorescence peaks. The Pi,n value of Region IV in the control assay increased to 96.99%, while that in the assays with ZVI-100, ZVI-1000 and NZVI were 68.67%, 74.10% and 82.29%, respectively. The reduced Pi,n reflected the effective conversion of biodegradable compounds to biogas with the supplementation of micrometer-scale or nano ZVI. In addition, the highest fluorescence intensity (26
056 au (Fig. S4†). The results implied that NZVI could promote the release of biodegradable compounds (including some unknown organic matters) to SFL, which was in agreement with the accumulation of acetate. Meanwhile, the Pi,n values of Region IV in the assays with ZVI-100 (76.46%) and ZVI-1000 (77.95%) were slightly lower than the control assay (80.67%), which might be due to the elevated methanogenesis (P < 0.05). In the intermediate stage, Region II and Region IV still contributed to the main fluorescence peaks. The Pi,n value of Region IV in the control assay increased to 96.99%, while that in the assays with ZVI-100, ZVI-1000 and NZVI were 68.67%, 74.10% and 82.29%, respectively. The reduced Pi,n reflected the effective conversion of biodegradable compounds to biogas with the supplementation of micrometer-scale or nano ZVI. In addition, the highest fluorescence intensity (26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 121 au) was still obtained in the assay with NZVI, which was consistent with the relatively high-concentration SCOD. With the consumption of biodegradable compounds, the Pi,n value of Region IV in the control assay decreased to 51.22% at the end of digestion, while Region III (fulvic acid-like organics) accounted for a higher proportion of 46.94%. In contrast, the biodegradable compounds in the assays with ZVI-100, ZVI-1000 and NZVI kept in a high level (74.19%, 71.73% and 72.76%, respectively). Furthermore, the Pi,n value of Region V in the assay with NZVI reached 14.72%, implying the accumulation of humic acid-like organics. From the perspective of metabolism, NZVI could promote the release of biodegradable compounds to SFL, while the substrate environment became adverse with the accumulation of humic acid-like organics at the end of digestion.
121 au) was still obtained in the assay with NZVI, which was consistent with the relatively high-concentration SCOD. With the consumption of biodegradable compounds, the Pi,n value of Region IV in the control assay decreased to 51.22% at the end of digestion, while Region III (fulvic acid-like organics) accounted for a higher proportion of 46.94%. In contrast, the biodegradable compounds in the assays with ZVI-100, ZVI-1000 and NZVI kept in a high level (74.19%, 71.73% and 72.76%, respectively). Furthermore, the Pi,n value of Region V in the assay with NZVI reached 14.72%, implying the accumulation of humic acid-like organics. From the perspective of metabolism, NZVI could promote the release of biodegradable compounds to SFL, while the substrate environment became adverse with the accumulation of humic acid-like organics at the end of digestion.
3.4 Bioavailability assessment of iron
The function of Fe0 could only be made through the reductive reaction via eqn (5), and the dissolved iron species was in the ferrous form (Fe2+) due to low ORP in the anaerobic environment. Thus Fe2+ release is an indicator to reflect the extent of its participation in the process.17 Fig. 6a showed that the smaller particle size was, the higher Fe2+ concentration could be obtained. Specifically, the rapid release of Fe2+ were observed on 3rd day in the assays with NZVI and ZVI-1000 with the values of 8.84 mg L−1 and 4.80 mg L−1, while the background level in the control assay was 2.05 mg L−1. It should be noted that the initial release of Fe2+ in the control assay was possibly due to the reduced pH (acidic environment) and intracellular material release.27 Subsequently the concentrations of Fe2+ in the assays with ZVI-100, ZVI-1000 and NZVI continued to increase with the final values of 5.78 mg L−1, 10.42 mg L−1 and 21.12 mg L−1 respectively, which was attributed to the continuous chemical corrosion of Fe0 in the AD system. Nevertheless, compared with the dosage of Fe0 in the system, the released Fe2+ in SFL was very low. The reason should be attributed to the formation of iron precipitates. Dissolved ZVI would react with phosphate, and it could be verified from the changes of phosphate concentration (Fig. 6b). Generally, the change of phosphate concentration in the AD system is a dynamic balance process, and the phosphate concentration would increase in the initial stage due to the rapid cell membrane decomposition and intracellular material release.25 Contrary to the trend of Fe2+ concentration, the highest level was observed in the control assay with a final concentration of 251.4 mg L−1, while that in the assays with micrometer-scale and nano ZVI was close to zero. The rapid capture of phosphate occurred in the assay with NZVI and no phosphate could be detected once NZVI was added, while 6 and 32 days were required for the assays with ZVI-100 and ZVI-1000. From this perspective, it could be another inhibitory factor since phosphorus is one of key nutrients for methanogens,31 and the rapid release of Fe2+ from NZVI hindered the phosphorus uptake. Thus, the synthetic effects of NZVI resulted in the relatively enhanced methane production along with a lag phase in the initial stage. | ||
| Fig. 6 (a) Variations of Fe2+ concentration in SFL during the AD process; (b) variations of TP concentration in SFL during the AD process. | ||
3.5 Pyrosequencing analysis of microbial community structure
High throughput pyrosequencing targeting 16S rRNA gene segments was employed to reveal the microbial community (bacteria and archaea) structure. The diversity estimators of Chao1, ACE and Shannon index and Good's coverage of sequencing for each sample were shown in Table 2. Each sample had a high coverage of sequences, which was more than 0.98 with no distinct difference. As a metric for species richness, Chao1 in the assay with NZVI (153) was lower than the assays with ZVI-100 and ZVI-1000, which integrated with the higher Shannon index (0.80) jointly implied that some bacteria were enriched selectively.| Sample | Effective reads | ACE | Chao1 | Shannon | Coverage |
|---|---|---|---|---|---|
| Control | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 343 343 |
225 | 246 | 0.60 | 0.99 |
| ZVI-100 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 508 508 |
202 | 199 | 0.62 | 0.98 |
| ZVI-1000 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 684 684 |
183 | 197 | 0.63 | 0.99 |
| NZVI | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 574 574 |
153 | 168 | 0.80 | 0.99 |
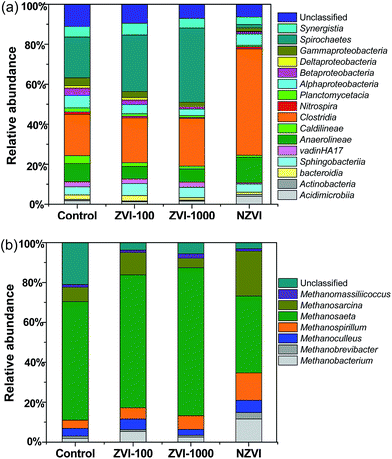
Distribution of bacterial sequences at the class level in each sample was shown in Fig. 7a. There were 16 classes with relative abundances of higher than 0.5% in at least one sample. Other classes were grouped into the unclassified group. Among them, Anaerolineae, Clostridia and Spirochaetes dominated in the reactors. Clostridia, as the common acid-forming bacteria, are recognized as effective hydrogen producers. The appearance of Clostridia could accelerate hydrolysis of the sludge, especially carbohydrate.32 Compared with the assays with no-ZVI (20.7%), ZVI-100 (22.4%) and ZVI-1000 (23.9%), the highest relative abundance of Clostridia was observed in the assay with NZVI (53.2%), which confirmed that NZVI could enhance hydrolysis-acidification. It was noteworthy that the detected H2 production in the assay with NZVI was not high, accompanied with the low CO2 production, implying H2 and CO2 were utilized effectively by hydrogenotrophic methanogens or homoacetogenic bacteria. Instead, Spirochaetes in the assay with NZVI accounted for only 1.6% of the total sequences and that in the assays with no-ZVI, ZVI-100 and ZVI-1000 were 20.4%, 28.3% and 37.1%, respectively. Spirochaetes are Gram-negative bacteria that live chemoheterotrophically in anaerobic environments and are generally known to ferment carbohydrates or amino acids into acetate, H2 and CO2.33 Therefore, the degradation of organic matters to precursors for methane production was jointly accomplished by the compatible collaborations of these bacteria in several trophic levels including hydrolysis, acetogenesis and syntrophic hydrogen-producing.
To fully display the effect of NZVI on microbial community structure, the relative abundances of methanogens (more than 0.5%) in each sample were identified at genus level (Fig. 7b). As the hydrogen scavengers, the higher relative abundances of Methanobacterium (11.6%), Methanobrevibacter (3.2%), Methanoculleus (6.2%) and Methanospirillum (13.7%) were observed in the assay with NZVI, indicating the important role of hydrogenotrophic methanogens. The strict acetotrophic genus Methanosaeta and facultative acetoclastic genus Methanosarcina were the two dominant genera in the reactors, which have been reported to be responsible for approximately 70% of the methane produced in AD process.34 Of which, the genus Methanosaeta includes exclusively acetotrophic species utilizing acetate as the sole substrate for metabolism. Except for the assay with NZVI, the relative abundances of Methanosaeta in the assays with ZVI-100 (66.6%) and ZVI-1000 (74.2%) were higher than the control assay (59.4%), which contributed to the enhanced methane production. In comparison, the highest relative abundance of Methanosarcina (22.6%) was observed in the assay with NZVI. Methanosarcina can produce methane from acetate, methanol, monomethylamine, dimethylamine, trimethylamine, H2/CO2, and CO, i.e. they are both acetoclastic and hydrogenotrophic methanogens.35 The results further provided evidence that NZVI could enhance the hydrogenotrophic methanogenesis, which was beneficial to methane production. Furthermore, Methanosarcina is seemingly more resistant to the non-stable conditions and the higher acetate concentration in the growth environment would favor the growth of Methanosarcina.36 It is suggested that NZVI induced the selective proliferation of Methanosarcina by facilitating the accumulation of acetate.
4. Conclusions
Results of this study confirm that NZVI has an important role on determining process performance and stability by directly influencing SFL and microbial community structure. Initially, NZVI addition negatively affected methane production, but after microbial adaptation, the system recovered with the enhanced methane production of 108.24 mL per gVS. Further examination of SFL indicated that NZVI could improve propionate degradation and hydrolysis-acidification processes, and the inhibitory effect in the initial stage was mainly due to the hindered VFAs utilization by acetoclastic methanogens. As expected, the acid-forming bacteria Clostridia and facultative acetoclastic genus Methanosarcina were enriched in the assay with NZVI, which were responsible to the enhanced hydrolysis-acidification and methanogenesis processes. The potential ability to create favorable substrate environment will provide support for further development of a more efficient NZVI-based AD system.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51178261), the Key project of Science and Technology Commission of Shanghai Municipality (12231202101, 14DZ1207306) and Open Funding Project of National Key Laboratory of Human Factors Engineering, Grant No. HF2012-K-05.Notes and references
- F. Gottschalk, T. Sonderer, R. W. Scholz and B. Nowack, Environ. Sci. Technol., 2009, 43, 9216–9222 CrossRef CAS PubMed.
- P. G. Tratnyek and R. L. Johnson, Nano Today, 2006, 1, 44–48 CrossRef.
- N. Kumar, M. Auffan, J. Gattacceca, J. Rose, L. Olivi, D. Borschneck, P. Kvapil, M. Jublot, D. Kaifas, L. Malleret, P. Doumenq and J. Y. Bottero, Environ. Sci. Technol., 2014, 48, 13888–13894 CrossRef CAS PubMed.
- S. Comba, A. D. Molfetta and R. Sethi, Water, Air, Soil Pollut., 2011, 215, 595–607 CrossRef CAS.
- B. I. Kharisov, H. R. Dias, O. V. Kharissova, V. M. Jiménez-Pérez, B. O. Perez and B. M. Flores, RSC Adv., 2012, 2, 9325–9358 RSC.
- S. C. N. Tang and I. M. C. Lo, Water Res., 2013, 47, 2613–2632 CrossRef CAS PubMed.
- B. Nowack and T. D. Bucheli, Environ. Pollut., 2007, 150, 5–22 CrossRef CAS PubMed.
- M. A. Kiser, P. Westerhoff, T. Benn, Y. Wang, J. Perez-Rivera and K. Hristovski, Environ. Sci. Technol., 2009, 43, 6757–6763 CrossRef CAS PubMed.
- B. Yu, D. L. Zhang, A. D. Shan, Z. Y. Lou, H. P. Yuan, X. T. Huang, W. X. Yuan, X. H. Dai and N. W. Zhu, RSC Adv., 2015, 5, 38538–38546 RSC.
- Y. B. Zhang, Y. H. Feng and X. Quan, Waste Manag., 2015, 38, 297–302 CrossRef CAS PubMed.
- M. H. Diao and M. S. Yao, Water Res., 2009, 43, 5243–5251 CrossRef CAS PubMed.
- Z. Xiu, Z. Jin, T. Li, S. Mahendra, G. Lowry and P. Alvarez, Bioresour. Technol., 2010, 101, 1141–1146 CrossRef CAS PubMed.
- C. Fajardo, L. T. Ortiz, M. L. Rodriguez-Membibre, M. Nande, M. C. Lobo and M. Martin, Chemosphere, 2012, 86, 802–808 CrossRef CAS PubMed.
- J. W. Chen, Z. M. Xiu, G. V. Lowry and P. J. J. Alvarez, Water Res., 2011, 45, 1995–2001 CrossRef CAS PubMed.
- J. Wang and J. Farrell, Environ. Sci. Technol., 2003, 37, 3891–3896 CrossRef CAS PubMed.
- Z. Y. Ji and Y. G. Chen, Environ. Sci. Technol., 2010, 44, 8957–8963 CrossRef CAS PubMed.
- Y. B. Zhang, Y. H. Feng, Q. L. Yu, Z. B. Xu and X. Quan, Bioresour. Technol., 2014, 159, 297–304 CrossRef CAS PubMed.
- K. W. Xu, H. Liu and J. Chen, Bioresour. Technol., 2010, 101, 2600–2607 CrossRef CAS PubMed.
- APHA, Standard methods for the examination of water and wastewater, 20st edn, 1998 Search PubMed.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, J. Biol. Chem., 1951, 193, 265–275 CAS.
- D. Herbert, P. Philipps and R. Strange, Methods Enzymol., 1971, 5, 265–277 Search PubMed.
- B. Yu, Z. Y. Lou, D. L. Zhang, A. D. Shan, H. P. Yuan, N. W. Zhu and K. H. Zhang, Bioresour. Technol., 2015, 179, 291–298 CrossRef CAS PubMed.
- W. Chen, P. Westerhoff, J. A. Leenheer and K. Booksh, Environ. Sci. Technol., 2003, 37, 5701–5710 CrossRef CAS PubMed.
- J. Zhang, Y. B. Zhang, X. Quan and S. Chen, Water Res., 2013, 47, 5719–5728 CrossRef CAS PubMed.
- Y. H. Feng, Y. B. Zhang, X. Quan and S. Chen, Water Res., 2014, 52, 242–250 CrossRef CAS PubMed.
- G. Y. Zhen, X. Q. Lu, Y. Y. Li, Y. Liu and Y. C. Zhao, Chem. Eng. J., 2015, 263, 461–470 CrossRef CAS.
- Y. Yang, J. L. Guo and Z. Q. Hu, Water Res., 2013, 47, 6790–6800 CrossRef CAS PubMed.
- A. Fey and R. Conrad, Appl. Environ. Microbiol., 2000, 66, 4790–4797 CrossRef CAS PubMed.
- L. Guo, M. M. Lu, Q. Q. Li, J. W. Zhang, Y. Zong and Z. L. She, Bioresour. Technol., 2014, 171, 22–28 CrossRef CAS PubMed.
- S. M. Wan, B. D. Xi, X. F. Xia, M. X. Li, D. D. Lv, L. Wang and C. H. Song, Bioresour. Technol., 2012, 123, 439–444 CrossRef CAS PubMed.
- H. Rudnick, S. Hendrich, U. Pilatus and K. H. Blotevogel, Arch. Microbiol., 1990, 154, 584–588 CrossRef CAS.
- S. Kim, S. Han and H. Shin, Int. J. Hydrogen Energy, 2004, 29, 1607–1616 CrossRef CAS.
- S. Lee, J. Park, H. Kang, Y. Lee, T. Lee and H. Park, Bioresour. Technol., 2013, 145, 25–32 CrossRef CAS PubMed.
- M. Ros, I. Franke-Whittle, A. Morales, H. Insam, M. Ayuso and J. Pascual, Bioresour. Technol., 2013, 136, 1–7 CrossRef CAS PubMed.
- J. Garcia, B. Patel and B. Ollivier, Anaerobe, 2000, 6, 205–226 CrossRef CAS PubMed.
- X. Guo, C. Wang, F. Sun, W. Zhu and W. Wu, Bioresour. Technol., 2014, 152, 420–428 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Transmission electron microscopic (TEM) images of NZVI; the cumulative H2 partial pressure vs. fermentation time; variations of pH vs. fermentation time; the EEM spectra of DOM during the AD process. See DOI: 10.1039/c6ra02591a |
| This journal is © The Royal Society of Chemistry 2016 |