SO3H-functionalized metal organic frameworks: an efficient heterogeneous catalyst for the synthesis of quinoxaline and derivatives†
Radoelizo S. Andriamitantsoa,
Jingjing Wang,
Wenjun Dong,
Hongyi Gao and
Ge Wang*
Beijing Key Laboratory of Function Materials for Molecule & Structure Construction, School of Materials Science and Engineering, University of Science and Technology Beijing, Beijing 100083, PR China. E-mail: gewang@mater.ustb.edu.cn; Tel: +86-10-62333765
First published on 1st April 2016
Abstract
An efficient alkylsulfonate functionalized metal organic framework (MOF), MIL-101-Cr–NH–RSO3H, has been successfully synthesized through a post-synthetic modification strategy of MIL-101-Cr–NH2 with 1,3-propanesultone reagent. The high surface area of the MIL-101-Cr–NH2 MOF guaranteed the high dispersion of –SO3H active species and the large pore size improved the contacting ability between the substrate and these active sites. The solid MIL-101-Cr–NH–RSO3H catalyst exhibited high catalytic performance in the preparation of quinoxaline derivatives by the condensation of benzene-1,2-diamines with 1,2-dicarbonyl compounds. Furthermore, the MIL-101-Cr–NH–RSO3H catalyst exhibits good stability, general applicability and excellent recycling performance.
Introduction
Quinoxaline skeletons and derivatives are a well known class of benzoheterocycles, which have biological activities as anticancer, kinase inhibitors, including antidepressant agents, antibacterial, anti-inflammatory and antiviral.1,2 Usually, the synthesis of quinoxaline is a condensation reaction between 1,2-diamine with 1,2 dicarbonyl compounds.3–12 Recently many homogeneous catalysts, such as Brønsted acids,13 metal salts14 and molecular iodine,15,16 have been developed for the preparation of quinoxaline and its derivatives. When acetic acid was used as the catalyst to synthesise quinoxaline and its derivatives, it was required to reflux in ethanol and stirred for 2–12 h while the product yields were only 34–85%.13 In the presence of Pd(OAc)2 or RuCl2-(PPh3)3 catalyst, the reaction was activated under TEMPO as the co-catalyst in toluene with a product yield of 83%.14 And in the case of iodine catalyst, the product yields could be reached up 85–95% at room temperature.15,16 However, several limitations in certain areas of quinoxaline synthesis still exist. Most of the studies involved a significant amount of catalyst, a high cost reagents, long reaction time,17 using dangerous and/or toxic solvents15,16 and unsatisfactory yields,13 which resulted in a great number of environmental and economic problems.18,19 Also, the main drawbacks of these catalysts were catalyst recycling and product separation. To overcome these problems, improved methods have been reported using heterogeneous catalyst, such as ZrO2/MxOy/MCM-41 (ref. 20) and Zn2+-K10-clay.17 However, the preparation of these catalysts required a complex procedure.Thus, studies of novel, simple and heterogeneous catalysts for the preparation of quinoxaline and its derivatives have gained growing attention due to their easy separation and facile recovery.21–24 Recently, MOFs have been involved intense research in heterogeneous catalysis.25 MOFs are porous nanomaterials constructed from metal containing cationic units and anionic organic linkers.25 The building components can be varied and functionalized. Functionalized MOFs show well-defined structures, high surface areas and tunable pore diameter that make them fascinating materials for applications in catalysis, gas separation, gas storage and sensing.25 Moreover, the functionalized MOFs can be further modified by post-synthetic modification (PSM), defined as covalently grafting the organic part with the functional species of parent MOFs.25–29 To develop a solid acid catalysts for the synthesis of quinoxaline skeleton and derivatives, typical amino-functionalized MOF (MIL-101-Cr–NH2) who showed large pores, good thermal stability and excellent chemical stability to water and common organic solvents were employed as support to graft the acid active species by PSM.30–36
In this study, a novel MOF-derived Brønsted acid catalyst, MIL-101-Cr–NH–RSO3H, has been synthesized conveniently using PSM method by the ring opening reaction of 1,3-propanesultone with MIL-101-Cr–NH2. The obtained MIL-101-Cr–NH–RSO3H as a solid acid catalyst was applied for the preparation of quinoxaline derivatives using benzene-1,2-diamines with 1,2-dicarbonyl compounds. The desired product has been obtained in a milder condition process with in higher yield during a shorter reaction time. The developed SO3H-based solid catalyst also showed easy recovery and excellent recycling stability for the catalytic reaction.
Experimental
Synthesis of MIL-101-Cr–NH2
MIL-101-Cr and MIL-101-Cr–NH2 were prepared using a reported procedure by electrophilic aromatic substitution.37,38 The preparation was carried out with nitration of MIL-101-Cr to form MIL-101-Cr–NO2, following a reduction of MIL-101-Cr–NO2 to form amino-MOF. MIL-101-Cr was synthesized through solvothermal route, chromium nitrate (2.0 g, 5.0 mmol) and terephthalic acid (0.83 g, 5.0 mmol) was dissolved in 24.0 mL of deionized water following the addition of 0.9 mL hydrofluorohydric acid (5.0 M), and stirring for 15 min. The mixture was introduced into Teflon-lined steel autoclave to heat at 220 °C for 8 h. The solid precipitate was removed by centrifugation, and the product was washed with deionized water three times and dried. The as-synthesized MIL-101-Cr was purified by treating 2.0 g of the sample in 40.0 mL aqueous mixture of ethanol (95% ethanol and 5% water) at 60 °C for 20 h. After cooling, the sample was washed and dried at 100 °C overnight.The MIL-101-Cr (∼0.60 g) was stirred overnight with 5.0 mL of HNO3 and 7.0 mL of H2SO4 in a water-ice bath between 5 and 10 °C. The obtained sample was filtered off, washed with water and dried at 100 °C overnight. Then synthesized MIL-101-NO2 (∼0.60 g) was suspended in 30.0 mL of HCl (37% v/v) solution containing SnCl2·2H2O (3.20 g, 14 mmol). The solution was stirred for 1 day at room temperature. The MIL-101-Cr–NH2 product was filtered off, washed with water and dried at 100 °C under vacuum.
Post-synthetic modification of MIL-101-Cr–NH2 to MIL-101-Cr–NH–RSO3H
MIL-101-Cr–NH2 (∼75 mg, 0.26 mmol NH2 equiv.) was dispersed in CHCl3 and then 2 equiv. (0.52 mmol) of 1,3-propanesultone was added into the mixture. The mixture was stirred overnight at room temperature, and then the sample was filtered and washed with CHCl3. The solvent was exchanged every 24 h for 2 days for the removal of any unreacted 1,3-propanesultone. The modified MIL-101-Cr–NH2 product were dried and heated at 60 °C for 8 h under vacuum.UiO-66-Zr–NH2 and UiO-66-Zr–NH–RSO3H were synthesized and characterized according to the reported method (see ESI†).39
General procedure for the condensation of benzene-1,2-diamine with benzil
210 mg (1.0 mmol) of benzil and 108 mg (1.0 mmol) of benzene-1,2-diamine were dissolved in 8.0 mL of ethanol separately in sonicator at room temperature. Both solutions were mixed in a beaker with appropriate amount of catalyst, and placed in a sonicator for few minutes at 45 °C. The reaction conversion was monitored by TLC. At the end of the reaction 15.0 mL of water was added into the solution and the mixture was cooled to ambient temperature. The solid catalyst was collected by centrifugation. The reaction was purified by flash chromatography over silica gel column (isocratic 5–10% ethyl acetate in petroleum ether) and the solvent was evaporated under reduced pressure to provide 2,3-diphenylquinoxaline, a white shiny solid (290.6 mg, 93% yield).For the recycling of MIL-101-Cr–NH–RSO3H catalyst, the catalyst was filtered off from the reaction and washed five times with water, then dried at 100 °C. The condensation reaction was performed using the recovered catalyst under the same reaction condition.
The hot filtration test of the catalyst was examined by isolating it from the mixture after 5 minutes of reaction during the condensation. Then the filtrate was subjected to keep run at the same reaction condition.
Characterization
The surface morphology of the samples was examined on a scanning electronic microscopy (SEM, ZEISS SUPRA55). The structure and phase of the samples was investigated by X-ray diffraction (XRD, M21X, Cu Kα radiation, λ = 0.154178 nm). The thermal decomposition of the samples were evaluated using a thermogravimetric analysis (TGA), NETZSCH STA 449 F3 Jupiter, under a N2 atmosphere, and the scanning rate was 5 °C min−1 in the temperature range of 40 °C to 800 °C. Fourier transformation infrared analysis (FT-IR) was recorded on Nicolet 6700 Spectrometer using KBr pellet method. All NMR spectra were recorded using Varian Unity Plus 400 (93.94 kG, 1H 400 MHz). Samples were commonly digested, and as an alternative solution, in CDCl3 or dimethyl sulfoxide-d6 for the pure organic compounds and in NaOD/D2O/DMSO-d6 for the digested MOFs samples. Nitrogen adsorption–desorption isotherms were measured at 77 K with a Micromeritics ASAP 2420 adsorption analyzer. The specific surface area was calculated using the Brunauer–Emmett–Teller (BET) method. The pore size distributions were derived from the adsorption branches of the isotherms by using the Barrett–Joyner–Halenda (BJH) model. Analytical thin layer chromatography (TLC) was performed using 0.25 mm silica gel 60-F254 plates. The chemical compositions were analysed by inductively coupled plasma-atomic emission spectrometry (ICP-AES, Varian 715-ES).Results and discussion
Catalysts characterization
The preparation procedure of MIL-101-Cr–NH–RSO3H solid Brønsted acid catalyst was shown in Scheme 1. Firstly, the nitration of MIL-101-Cr to yield MIL-101-Cr–NO2 was carried out by the mixed nitrating acid (concentrated HNO3/concentrated H2SO4) and then MIL-101-Cr–NH2 was obtained by subsequent reduction using SnCl2/concentrated HCl.37,38 The PSM of the amino-MOF was carried out by the ring opening reactions between 1,3-propanesultone by the amine-bearing MIL-101-Cr–NH2 to generate alkyl sulfonic acid groups (Scheme 1c).SEM images of MIL-101-Cr–NH2 and MIL-101-Cr–NH–RSO3H were presented in Fig. 1. The SEM micrographs showed that MIL-101-Cr–NH2 had a double-side pyramid crystalline structure (octahedral shape) and the particles were well dispersed (Fig. 1a). SEM images of MIL-101-Cr–NH–RSO3H (Fig. 1b) showed the identical and maintained structural morphology of MIL-101-Cr–NH2, indicating the strong chemical stability of the MIL-101-Cr–NH2 support during the PSM.
Crystal structures of MIL-101-Cr sample before and after PSM were characterized by XRD measurements range from 2θ = 2–15° (Fig. 2). The main diffraction peaks of the MIL-101-Cr sample at 2θ = 3.25, 5.10, 8.41, 9.02 and the relative diffraction intensities corresponded well to the literature data,37 which indicated that the desired MOF support was obtained (Fig. 2a). After nitration, reduction and the PSM, the crystal structure of MIL-101-Cr was maintained according to the XRD pattern of MIL-101-Cr–NO2 (Fig. 2b), MIL-101-Cr–NH2 (Fig. 2c) and MIL-101-Cr–NH–RSO3H (Fig. 2d) respectively. However, the crystallinity of MIL-101-Cr–NO2, MIL-101-Cr–NH2 and MIL-101-Cr–NH–RSO3H samples was less integrated in comparison with MIL-101-Cr, which may be due to the successful PSM of MIL-101-Cr. Likewise, the SEM image and the XRD pattern of UiO-66-Zr–NH2 (Fig. S1a and S2a†) was in agreement with the literature.40 And after PSM with 1,3-propanesultone, similar morphology and crystallinity structure to the parent MOF was shown in Fig. S1b and S2b,† respectively.
 | ||
| Fig. 2 XRD patterns of: (a) MIL-101-Cr, (b) MIL-101-Cr–NO2, (c) MIL-101-Cr–NH2 and (d) MIL-101-Cr–NH–RSO3H. | ||
TGA curves of MIL-101-Cr–NO2, MIL-101-Cr–NH2 and MIL-101-Cr–NH–RSO3H obtained under N2 atmospheres were shown in Fig. 3. The thermal stability of each sample depended on its functionality. The TGA curve of as-synthesized MIL-101-Cr–NO2 material showed the decomposition of the frameworks started from ∼400 °C up to 550 °C indicating its good thermal stability. The MIL-101-Cr–NH2 also showed good thermal stability with a slightly decomposition temperature approximately 330 °C in comparison to MIL-101-Cr–NO2. MIL-101-Cr–NH–RSO3H sample showed significant weight losses below 280 °C, which may be attributed to the successful incorporation of alkyl sulfonic group.41,42
To demonstrate the successful modification of the functional groups, the samples were analyzed by means of FT-IR characterization. The FT-IR spectrum of as-synthesized MIL-101-Cr (Fig. 4a) was in good agreement to previous report.37 The FT-IR spectrum of MIL-101-Cr–NO2 (Fig. 4b) revealed the successful nitration of MIL-101-Cr, due to the presence of the peaks characteristic for the asymmetric for –NO2 stretching and the C–N bonds at 1537 and 1360–1290 cm−1 respectively.38 Clear proof of amino group formation was observed by the absorption band of the asymmetric and symmetric N–H stretching vibration appeared at 3490 and 3378 cm−1 (Fig. 4c), which were assigned to the characteristic peak of the amine stretching vibration in MIL-101-Cr–NH2.32 And the C–N stretching vibration was clearly visible at the same region then MIL-101-Cr–NO2. To prove the successful functionalization with alkyl sulfonic acid, the FT-IR characterization of MIL-101-Cr–NH–RSO3H was also performed and was presented in Fig. 4d. The FT-IR spectrum of the MIL-101-Cr–NH–RSO3H showed several new bands in the region 1240 cm−1 which were assigned to the asymmetric and symmetric stretching of the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional group and at 1040 cm−1 assigned to S–O stretching vibration.39,43 And the FT-IR spectrum of MIL-101-Cr–NH–RSO3H showed the characteristic wide peak of the –OH group which was centred at 3400 cm−1.39
O functional group and at 1040 cm−1 assigned to S–O stretching vibration.39,43 And the FT-IR spectrum of MIL-101-Cr–NH–RSO3H showed the characteristic wide peak of the –OH group which was centred at 3400 cm−1.39
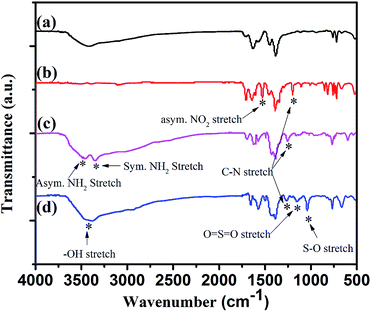 | ||
| Fig. 4 FT-IR spectra of: (a) MIL-101-Cr, (b) MIL-101-Cr–NO2, (c) MIL-101-Cr–NH2 and (d) MIL-101-Cr–NH–RSO3H. | ||
Further, the successful modification of the functional groups and the modification ratio of the alkyl sulfonic moiety were analyzed by 1H NMR spectroscopy. 1H NMR spectra of MIL-101-Cr, MIL-101-Cr–NO2 and MIL-101-Cr–NH–RSO3H were shown in Fig. 5. Samples were digested using NaOH and dissolved in D2O/NaOD solution prior to NMR analysis. 1H NMR spectrum of digested MIL-101-Cr–NH2 (Fig. 5b) confirmed the formation of the amine compound by the appearance of the aromatic signals associated with the phenyl protons substituted with the amine at 7.00–7.02, 7.36 and 7.75–7.77 ppm.42,44 Moreover, the 1H NMR spectrum of the digested MIL-101-Cr–NH–RSO3H (Fig. 5c) showed singlet resonances associated with the alkyl protons at 2.59–2.85 ppm and appearance of two singlet resonance at 7.90 and 8.08 ppm,39 and indicated that 55% of the amine groups were modified with alkylsulfonate groups.
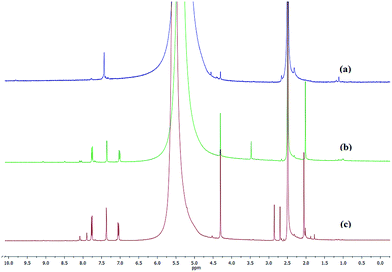 | ||
| Fig. 5 1H-NMR spectra of the digested samples: (a) MIL-101-Cr, (b) MIL-101-Cr–NH2 and (c) MIL-101-Cr–NH–RSO3H. | ||
Nitrogen adsorption–desorption was performed to evaluate the surface area, pore volume and pore structure of the samples (Fig. 6). All the samples featured the same type IV isotherm, which indicated the pore structure was well retained after functionalization. The surface areas of MIL-101-Cr–NH2 and MIL-101-Cr–NH–RSO3H was determined by the BET method based on nitrogen adsorption–desorption isotherms. The total pore volumes were obtained from the volume of N2 adsorption at P/P0 = 0.985. The BET surface area and pore volume of as-synthesized MIL-101-Cr–NH2 sample were 2280 m2 g−1 and 2.55 cm3 g−1 respectively. After PSM of the MIL-101-Cr–NH2, MIL-101-Cr–NH–RSO3H showed a calculated surface area 615.6 m2 g−1 and a pore volume of 0.71 cm3 g−1. The obvious reduction in surface area as well as pore volume demonstrated that the pores of MIL-101-Cr–NH2 were occupied by the bulky alkylsulfonate groups.45
Catalytic study
The catalytic activity of alkylsulfonate functionalized MOF catalyst was investigated by the condensation reaction of benzene-1,2-diamine with benzil under the sonication (Table 1). The blank control experiment in the absence of catalyst gave no product in 300 min (Table 1, entry 1). After adding Brønsted acid catalyst, such as p-toluenesulfonic acid (Table 1, entry 2), 2,3-diphenylquinoxaline was formed which indicated that the Brønsted acid could be conductive to the condensation reaction. Therefore, the amino group in MIL-101-Cr–NH2 (Table 1, entry 3) and UiO-66-Zr–NH2 (Table 1, entry 4) would inhibit the condensation of benzene-1,2-diamine with benzil. In the homogeneous systems, 2,3-diphenylquinoxaline with a yield of 93% was obtained within 8 min which indicated that the catalyst was catalytically efficient for the condensation reaction. However, the recovery and reusability of the catalyst from the reaction media is still an important problem. When the MIL-101-Cr–NH–RSO3H sample was used as the catalyst, the yield of 2,3-diphenylquinoxaline reached up to 93% in 12 min (Table 1, entry 5). The enhanced catalytic efficiency for the condensation reaction may be ascribed to the high dispersion of active catalytic sites at the pore walls of the MOF support. Interestingly, similar result also was observed when the UiO-66-Zr–NH–RSO3H samples were used as catalysts (Table 1, entry 6). The yield of 2,3-diphenylquinoxaline for the MIL-101-Cr–NH–RSO3H catalyst was higher than that for the UiO-66-Zr–NH–RSO3H catalyst, which may be due to the different pores structures between the catalysts. As shown in Fig. S3,† the surface area and pore volume of UiO-66-Zr–NH2 MOF was 1140 m2 g−1 and 0.36 cm3 g−1 respectively. The condensation of benzene-1,2-diamine with benzil was also carried out using the sulfonated-polystyrene (SPS) catalyst. The SPS catalyst was obtained by simple sulfonation of commercial expanded polystyrene (PS) according to the reported procedures.46,47 FT-IR analysis of PS and SPS catalyst were shown in Fig. S4.† The quinoxaline product was obtained after 25 min of reaction with an isolated yield of 74% (Table 1, entry 7). The result showed that the yield of the product was lower compared to MIL-101-Cr–NH–RSO3H, which may be due to the rigidity and the low surface areas of the SPS. The high crosslink contents of these acid resins make a difficult accessibility and diffusion of the substrates.48 If the reaction occurred only on the external surface of the catalysts, we would see little difference in the yield and reaction time between using MIL-101-Cr–NH–RSO3H and SPS catalyst. However, there were some differences observed in the yield of the product, which may be due to that the large pores of the support MIL-101-Cr–NH2 improve the contacting ability between the substrate and the active centers –SO3H and ensure the evacuation of product molecules from the network. The above result may suggest that the reaction occurs on both external and internal surfaces.49,50| Entry | Solvent | Catalyst | Time (min) | Yield (%) |
|---|---|---|---|---|
a A solution of benzene-1,2-diamine (1.0 mmol) and benzil (1.0 mmol) separately in the appropriate solvent were mixed in the presence of catalysts (13 mol% –SO3H). The progress of the reaction was monitored by TLC using Pet. ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc (90 EtOAc (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10).b 0.1 mmol based on the active site of –NH2.c 0.2 mmol of catalyst. 10).b 0.1 mmol based on the active site of –NH2.c 0.2 mmol of catalyst. |
||||
| 1 | Methanol | — | 300 | — |
| 2c | Methanol | p-Toluenesulfonic acid | 8 | 93 |
| 3b | Methanol | MIL-101-Cr–NH2 | 300 | — |
| 4b | Methanol | UiO-66-Zr–NH2 | 300 | — |
| 5 | Methanol | MIL-101-Cr–NH–RSO3H | 12 | 93 |
| 6 | Methanol | UiO-66-Zr–NH–RSO3H | 15 | 85 |
| 7c | Methanol | SPS | 25 | 74 |
| 8 | Ethanol | MIL-101-Cr–NH–RSO3H | 18 | 90 |
| 9 | Isopropanol | MIL-101-Cr–NH–RSO3H | 18 | 75 |
| 10 | Dichloromethane | MIL-101-Cr–NH–RSO3H | 25 | 15 |
| 11 | Acetonitrile | MIL-101-Cr–NH–RSO3H | 40 | 30 |
| 12 | Dimethylformamide | MIL-101-Cr–NH–RSO3H | 48 | 21 |
| 13 | Dimethyl sulfoxide | MIL-101-Cr–NH–RSO3H | 36 | 22 |
Solvent may serve two major purposes, namely, it dissolves the reactants and participates as a source of an acid (proton), base (removing protons), or as a nucleophile in the condensation reaction of benzene-1,2-diamine with benzil.51–53 The model reaction was performed in various solvents and the results were summarized in Table 1. When a polar-protic solvent, such as ethanol, methanol and propanol, was employed in the reaction respectively (Table 1, entry 5,8–9), the yield of the product was 93%, 90% and 75% respectively. When non-polar solvent like dichloromethane was employed in the reaction, the yield of 2,3-diphenylquinoxaline was very low and also the reaction was not completed even after 25 min of sonication (Table 1, entry 10). Furthermore, polar aprotic solvents like acetonitrile, dimethylformamide and dimethyl sulfoxide provided lower yield and acquired longer reaction time compared to polar-protic solvents (Table 1, entry 11–13). The above results indicated that the polar-protic solvent was beneficial to the enhancement of the yield of 2,3-diphenylquinoxaline, which may be due to that the hydrogen bonding between these polar-protic solvents can serve as a source of protons (H+) similar to the –SO3H groups of the catalyst to activate benzene-1,2-diamine through protonation for further reaction with benzil.51 A plausible mechanism for the condensation of benzene-1,2-diamine with benzil was provided in Fig. S5.† On the basis of this mechanism, benzil reacts efficiently with two nucleophilic groups after electrophile activation by MIL-101-Cr–NH–RSO3H catalyst.51,54 Because of the two nucleophilic groups of the diamine is part of the same molecule, a cyclic intermediate product is formed by nucleophilic attack. The lone pair of electrons on the adjacent nitrogen, by resonance with benzene ring, performs as carbanion and acts as the driving force for the departure of –OH group, followed closely by abstraction of proton on nitrogen. By successive elimination of H2O, the cyclocondensation take places to produce the heterocycles, 2,3-diphenylquinoxaline and the regeneration of MIL-101-Cr–NH–RSO3H catalyst.
The influence of the catalyst concentration of MIL-101-Cr–NH–RSO3H on benzene-1,2-diamine condensation with benzil was investigated and shown in Fig. 7. 0.7 mol%, 1.3 mol%, 3.9 mol%, 6.5 mol% and 13 mol% amount of catalyst was performed respectively. It was seen that the increase in amount of catalyst gradually increased the yield of product. Lower amount of MIL-101-Cr–NH–RSO3H, 0.7 mol% and 1.3 mol%, provided relatively low yield of 65% and 72% respectively. With the increasing of the MIL-101-Cr–NH–RSO3H amount to 3.9 mol%, 6.5 mol% and 13 mol%, the yield of 2,3-diphenylquinoxaline was maintained at 93%. As a result, catalyst loading of 3.9 mol% was chosen as the optimal reaction condition for further catalytic studies. Therefore, the optimized catalytic condition for the synthesis of quinoxaline involved that 1 mmol of the substrate, 3.9 mol% MIL-101-Cr–NH–RSO3H catalyst based on –SO3H, and methanol as an efficient solvent at 45 °C with sonication for 14 minutes. Such a catalytic system is more efficient and eco-friendly than the reported results (Table 2).
 | ||
| Fig. 7 Influence of the MIL-101-Cr–NH–RSO3H catalyst concentration on the yield of 2,3-diphenylquinoxaline. | ||
| Entry | Catalyst | Time (min) | Solvent | Temperature (°C) | Yielda (%) | References |
|---|---|---|---|---|---|---|
| a Isolated yield (%). | ||||||
| 1 | Heteropoly acid | 5 | Acetic | 50 | 98 | 12 |
| 2 | Cellulose sulfuric acid | 60 | Ethanol | 50 | 93 | 55 |
| 3 | Zn2+-K10-clay | 24 h | Water/ethanol | 50 | 89 | 17 |
| 4 | ZrO2/MxOy/MCM-41 | 120 | Acetonitrile | 50 | 97 | 20 |
| 5 | Y-Zeolite | 60 | Water/ethanol | 50 | 80 | 6 |
| 6 | MIL-101-Cr–NH–RSO3H | 14 | Methanol | 45 | 93 | This work |
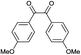
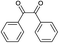
With the optimal reaction condition, the syntheses of quinoxaline derivatives with different substituents on both reactants were investigated to demonstrate the general applicability of the MIL-101-Cr–NH–RSO3H catalyst. As a result, when biacetyl was used as substrate, 91% yield of product could be obtained within 5 min (Table 3, entry 1). When a benzil substituted with a strong withdrawing (Table 3, entry 2) and electron-donating group (Table 3, entry 3) was used, the reaction proceeded slowly than that of benzil (Table 3, entry 4). Also, we explored the general applicability of the MIL-101-Cr–NH–RSO3H catalyst using benzene-1,2-diamine with different substituents as substrate. It was observed that the reaction proceeded very efficiently with 4-methylbenzene-1,2-diamine (Table 3, entry 5). The results showed that the electronic nature of the substituents in the aromatic ring of benzil and the benzene-1,2-diamine had no significant effect on the yields of the reaction. However, compare to the above results, the condensation of (3,4-diaminophenyl)phenylmethanone with benzil (Table 3, entry 6) and 1,2-bis(4-methoxyphenyl)ethane-1,2-dione (Table 3, entry 7) gave lower yield 81–82% and the reaction required longer reaction time 20–24 minutes. The distinct yields suggested that the reactivity of the reaction was highly dependent on the steric hindrance not the electronic properties of the aromatic ring of the reactants.
| Entry | Diamine | Substrate | Product | Time (min) | Yield (%) |
|---|---|---|---|---|---|
a A solution of 1,2-diamine (1.0 mmol) and the appropriate 1,2-dicarbonyl compounds (1.0 mmol) separately in methanol were mixed in the presence of catalysts (3.9 mol% –SO3H). The progress of the reaction was monitored by TLC using Pet. ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc (90 EtOAc (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 10). |
|||||
| 1 |  |
 |
 |
5 | 91 |
| 2 |  |
 |
 |
15 | 85 |
| 3 |  |
 |
 |
17 | 87 |
| 4 | 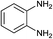 |
 |
 |
12 | 93 |
| 5 |  |
 |
 |
15 | 88 |
| 6 |  |
 |
 |
20 | 82 |
| 7 | 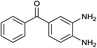 |
 |
 |
24 | 81 |
The sulfur content of the fresh MIL-101-Cr–NH–RSO3H catalyst was determined using inductively coupled plasma-atomic emission spectrometry (ICP-AES). ICP-AES analysis showed the presence of 56.7 mmol g−1 sulfur in the fresh MIL-101-Cr–NH–RSO3H catalyst.
A hot filtration test was performed by separating the MIL-101-Cr–NH–RSO3H catalyst from the reaction mixture after 5 minutes in the first cycle of reaction. The recovered MIL-101-Cr–NH–RSO3H was collected and then dried at 100 °C. ICP-AES analysis of the recovered MIL-101-Cr–NH–RSO3H indicated the presence of 55.2 mmol g−1 sulfur, which showed that there was 2.6 mol% sulfur leached into the filtrate. This may led to the slight increase of the yield to 59% from 55% after the hot filtration test (Fig. 8).
 | ||
| Fig. 8 Hot filtration test for MIL-101-Cr–NH–RSO3H catalyzed the condensation of benzene-1,2-diamine with benzil in methanol. | ||
In order to evaluate the reusability of MIL-101-Cr–NH–RSO3H catalyst, the condensation reaction was performed using the recovered catalyst under optimized reaction conditions. The catalyst was filtered after the reaction and washed five times with ethanol, then dried at 100 °C, and reused in the next run of the reaction. The catalyst remained active during each recycle and gave 79% yield of product even after five recycles (Fig. 9), which showed the good recycling ability of MIL-101-Cr–NH–RSO3H catalyst. The MIL-101-Cr–NH–RSO3H catalyst after the fifth run was analyzed by XRD and FT-IR. The XRD patterns of the recovered MIL-101-Cr–NH–RSO3H catalyst (Fig. S6†) showed the entire typical peak characteristic of the fresh catalyst, which confirmed the chemical stability of the MIL-101-Cr–NH–RSO3H catalyst. In comparison, the FT-IR spectrum of the recovered MIL-101-Cr–NH–RSO3H catalyst showed similar absorbing band characteristic to the fresh MIL-101-Cr–NH–RSO3H (Fig. S7†), suggesting high stability of supported –SO3H heterogeneous catalyst.
 | ||
| Fig. 9 Recycling of the MIL-101-Cr–NH–RSO3H catalyst in the condensation of benzene-1,2-diamines with benzil. | ||
Conclusions
In summary, a heterogeneous Brønsted acid catalyst, MIL-101-Cr–NH–RSO3H, was developed through a post-synthetic modification of MIL-101-Cr–NH2 using 1,3-propanesultone. The high surface area and large pore size of the parent MIL-101-Cr–NH2 MOF not only ensured enough contact between the substrate and the active center of –SO3H in the framework but also promoted the evacuation of product molecules from the network. The as-synthesized MIL-101-Cr–NH–RSO3H catalyst provided the high catalytic activity for the condensation of 1,2-diamines with benzil. The MIL-101-Cr–NH–RSO3H catalyst also showed good reusability and general applicability towards various kinds of 1,2-diamines and 1,2 dicarbonyl compounds. The incorporation strategy of –SO3H active species in the framework of MOF support provides a simple, low-cost and highly efficient approach for the synthesis of heterogeneous catalysts.Acknowledgements
This work was supported by National Natural Science Foundation of China (863 Program, no. 2013AA031702) and the Co-building Special Project of Beijing Municipal Education.Notes and references
- W. He, M. R. Meyers, B. Hanney, A. Spada, G. Blider, H. Galzeinski, D. Amin, S. Needle, K. Page, Z. Jayyosi and H. Perrone, Bioorg. Med. Chem. Lett., 2003, 13, 3097 CrossRef CAS PubMed.
- A. Dell, D. H. William, H. R. Morris, G. A. Smith, J. Feeney and G. C. K. Roberts, J. Am. Chem. Soc., 1975, 97, 2497 CrossRef CAS PubMed.
- Z. Zhao, D. D. Wisnoski, S. E. Wolkenberg, W. H. Leister, Y. Wang and C. W. Lindsley, Tetrahedron Lett., 2004, 45, 4873 CrossRef CAS.
- A. Heshmatollah, T. Mahmood, N. Mohammad and B. Saeed, World Appl. Sci. J., 2013, 22, 1711 Search PubMed.
- S. A. Raw, C. D. Wilfred and R. J. K. Taylor, Org. Biomol. Chem., 2004, 2, 788 CAS.
- C. Venkatesh, B. Singh, P. K. Mahata and H. Junjappa, Org. Lett., 2005, 7, 2169 CrossRef CAS PubMed.
- T. K. Huang, R. Wang, L. Shi and X. X. Lu, Catal. Commun., 2008, 9, 1143 CrossRef CAS.
- M. M. Heravi, K. Bakhtiari, F. F. Bamoharram and M. H. Tehrani, Monatsh. Chem., 2007, 138, 465 CrossRef CAS.
- M. M. Heravi, K. Bakhtiari, M. H. Tehrani, N. M. Javadi and H. A. Oskooie, ARKIVOC, 2006, 16, 253 Search PubMed.
- S. V. More, M. N. V. Sastry and C. F. Yao, Green Chem., 2006, 8, 91 RSC.
- L. M. Wang, J. J. Liu, H. Tian and C. Qian, Synth. Commun., 2004, 34, 1349 CrossRef CAS.
- H. R. Darabi, S. Mohandessi, K. Aghapoor and F. Mohsenzadeh, Catal. Commun., 2007, 8, 389 CrossRef CAS.
- D. J. Brown, Quinoxalines: Supplement II, in The Chemistry of Heterocyclic Compounds, ed. E. C. Taylor and P. Wipf, John Wiley & Sons, New Jersey, 2004 Search PubMed.
- R. S. Robinson and R. J. K. Taylor, Synlett, 2005, 6, 1003 Search PubMed.
- R. S. Bhosale, S. R. Sarda, S. S. Ardhapure, W. N. Jadhav, S. R. Bhusare and R. P. Pawar, Tetrahedron Lett., 2005, 46, 7183 CrossRef CAS.
- S. V. More, M. N. V. Sastry, C. C. Wang and C. F. Yao, Tetrahedron Lett., 2005, 46, 6345 CrossRef CAS.
- A. Dhakshinamoorthy, K. Kanagaraj and K. Pitchumani, Tetrahedron Lett., 2011, 52, 69 CrossRef CAS.
- D. Villemin and B. Martin, Synth. Commun., 1995, 25, 2319 CrossRef CAS.
- J. C. Feng, Y. Liu and Q. H. Meng, Synth. Commun., 1998, 28, 193 CrossRef CAS.
- S. Ajaikumar and A. Pandurangan, Appl. Catal., A, 2009, 357, 184 CrossRef CAS.
- J. S. Seo, D. Wand, H. Lee, S. I. Jun, J. Oh, Y. Jeon and K. Kim, Nature, 2000, 404, 982 CrossRef CAS PubMed.
- J. M. Notestein and A. Katz, Chem.–Eur. J., 2006, 12, 3954 CrossRef CAS PubMed.
- R. H. Grubbs, CHEMTECH, 1977, 7, 512 CAS.
- S. Desportes, D. Steinmetz, M. Hémati, K. Philippot and B. Chaudret, Powder Technol., 2005, 157, 12 CrossRef CAS.
- O. M. Yaghi and H. Li, J. Am. Chem. Soc., 1995, 117, 10401 CrossRef CAS.
- M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469 CrossRef CAS PubMed.
- M. Eddaoudi, H. Li and O. M. Yaghi, J. Am. Chem. Soc., 2000, 122, 1391 CrossRef CAS.
- J. S. Costa, P. Gamez, C. A. Black, O. Roubeau, S. J. Teat and J. Reedijik, Eur. J. Inorg. Chem., 2008, 10, 1551 CrossRef.
- Z. Q. Wang and S. M. Cohen, J. Am. Chem. Soc., 2007, 129, 12368 CrossRef CAS PubMed.
- S. H. Jhung, J. H. Lee, J. W. Yoon, C. Serre, G. Férey and J. S. Chang, Adv. Mater., 2007, 19, 121 CrossRef CAS.
- A. Schaate, P. Roy, A. Godt, J. Lippke, F. Waltz, M. Wiebcke and P. Behrens, Chem.–Eur. J., 2011, 17, 6643 CrossRef CAS PubMed.
- M. Kandiah, M. H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. Quadrelli, F. Bonino and K. P. Lillerud, Chem. Mater., 2010, 22, 6632 CrossRef CAS.
- W. Morris, C. J. Doonan and O. M. Yaghi, Inorg. Chem., 2011, 50, 6853 CrossRef CAS PubMed.
- F. Vermoortele, R. Ameloot, A. Vimont, C. Serre and D. De Vos, Chem. Commun., 2011, 47, 1521 RSC.
- S. J. Garibay and S. M. Cohen, Chem. Commun., 2010, 46, 7700 RSC.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850 CrossRef PubMed.
- G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé and I. Margiolaki, Science, 2005, 309, 2040 CrossRef PubMed.
- S. Bernt, V. Guillerm, C. Serre and N. Stock, Chem. Commun., 2011, 47, 2838 RSC.
- Y. Luan, N. N. Zheng, Y. Qi, J. Yu and G. Wang, Eur. J. Inorg. Chem., 2014, 26, 4268 CrossRef.
- M. Pintado-Sierra, A. M. Rasero-Almansa, A. Corma, M. Iglesias and F. Sanchez, J. Catal., 2013, 299, 137 CrossRef CAS.
- A. G. Meike, G. Sylvain, S. B. Tobias, K. Johannes, M. G. Andreas, W. Silke, B. Stefan and K. Wolfgang, Dalton Trans., 2015, 44, 16802 RSC.
- Y. Luan, Y. Qi, H. Y. Gao, S. A. Radoelizo, N. N. Zheng and G. Wang, J. Mater. Chem. A., 2015, 3, 17320 CAS.
- P. Atorngitjawat, R. J. Klein and J. Runt, Macromolecules, 2006, 39, 1815 CrossRef CAS.
- J. G. Vitillo, M. Savonnet, G. Ricchiardi and S. Bordiga, ChemSusChem, 2011, 4, 1281 CrossRef CAS PubMed.
- J. J. Wang, M. Yang, W. J. Dong, Z. K. Jin, J. Tang, S. Fan, Y. F. Lu and G. Wang, Catal. Sci. Technol., 2016, 6, 161 Search PubMed.
- X. W. Zhang, W. J. Dong, Y. Luan, M. Yang, L. Tan, Y. G. Guo, H. Y. Gao, Y. H. Tang, R. Dang, J. Li and G. Wang, J. Mater. Chem. A., 2015, 3, 4266 CAS.
- M. Yang, Y. G. Guo, Q. Wu, Y. Luan and G. Wang, Polymer, 2014, 55, 1948 CrossRef CAS.
- R. Stern and G. Hillion, Chemical Abstracts, 1990, 113, 58504 Search PubMed.
- Y. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450 RSC.
- M. E. Davis, Acc. Chem. Res., 1993, 26, 111 CrossRef CAS.
- C. K. Z. Andrade and L. M. Alves, Curr. Org. Chem., 2005, 9, 195 CrossRef CAS.
- D. C. Cronaur, D. M. Jewell, Y. T. Shah and R. J. Modi, Ind. Eng. Chem. Fundam., 1979, 18, 153 Search PubMed.
- R. Gani, C. Jiménez-González and D. J. C. Constable, Comput. Chem. Eng., 2005, 29, 1661 CrossRef CAS.
- T. K. Huang, R. Wang, L. Shi and X. Lu, Catal. Commun., 2008, 9, 1143 CrossRef CAS.
- A. Shaabani, A. H. Rezayan, M. Behnam and M. Heidary, C. R. Chim., 2009, 12, 1249 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and PSM of UiO-66-Zr–NH2, sulfonation of polystyrene; SEM, XRD, nitrogen adsorption–desorption isotherms of UiO-66-Zr–NH2 and UiO-66-Zr–NH–RSO3H; FT-IR and TGA of SPS and XRD, FTIR of the recovered catalyst. See DOI: 10.1039/c6ra02575g |
| This journal is © The Royal Society of Chemistry 2016 |






