Synthesis and characterization of N-heterocyclic carbene-palladium(ii) chlorides-1-methylindazole and -1-methylpyrazole complexes and their catalytic activity toward C–N coupling of aryl chlorides†
Abstract
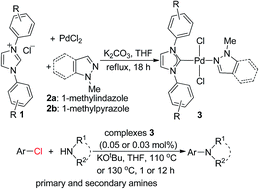
A series of N-heterocyclic carbene-palladium(II) chlorides-1-methylindazole and -1-methylpyrazole complexes was successfully synthesized and fully characterized by X-ray single crystal diffraction. In addition, initial investigations of their catalytic activity showed that they were efficient catalysts in the C–N coupling of primary and secondary amines with aryl chlorides at low catalyst loadings.

 Please wait while we load your content...
Please wait while we load your content...