Flower-like polyaniline–NiO structures: a high specific capacity supercapacitor electrode material with remarkable cycling stability
Bangning Suna,
Xinping Hea,
Xijin Lenga,
Yang Jiangb,
Yudong Zhaoa,
Hui Suo*a and
Chun Zhaoa
aState Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, Changchun, 130012, P. R. China. E-mail: suohui@jlu.edu.cn
bProduction Center of China Mobile Communications Corporation, Changchun, Jilin 130103, P. R. China. E-mail: 517579103@qq.com
First published on 13th April 2016
Abstract
In this study, we report a binder-free in situ approach to synthesize a polyaniline–NiO composite on a nickel foam as a supercapacitor electrode. A high specific capacitance of 2565 F g−1 at a current density of 1 A g−1 is achieved and it remains at 70% when increasing the current density by 10 times. Flower-like nanostructures are identified and offer a structural benefit for high levels of redox. Furthermore, this electrode also exhibits excellent cycling stability with a high retention of 100% for almost 5000 cycles.
Introduction
With the widespread energy crisis, an urgent need to develop renewable sources and improve energy storage exists worldwide. This leads to an explosive growth of research in these fields and supercapacitors prove to be a most promising candidate among energy storage devices.1–3 Supercapacitors can be utilized in a number of everyday electronic appliances such as car starter motors and provide better performance than a traditional battery.4,5 The electrical double-layer capacitors (EDLCs) and pseudocapacitors are the two main types of supercapacitor.6 In particular, these two types of supercapacitors almost co-exist in all capacitive devices. We need to identify which part contributes more during the charge and discharge processes. Physical processes occur when EDLCs store charge—when the electrolyte ions are absorbed onto the electrode/electrolyte interface by electrostatic force.7 The diffusion and accumulation processes of an electrostatic charge act so rapidly that extraordinarily high power density can be provided by EDLCs. Moreover, the energy density of EDLCs largely depends on the morphology of the electrode that dominates the electrochemically active specific surface area.8–10 Furthermore, restricted by the surface microtextures design technology and the fabrication procedure, the energy densities of EDLCs were always limited below 10 W h kg−1.11,12Considering the energy density, pseudocapacitors (using electrochemical storage of electrical energy by faradaic processes) provide a better choice owing to their high capacitance derived from reversible, fast redox reactions.13,14 Decent electronic conductivity, abundant electroactive sites and high stability in electrolyte are the fundamental prerequisites for a high performance pseudocapacitor electrode.13 Furthermore, considering practical applications, this electrode material should also possess the characteristics of low cost and low toxicity. This is what impedes the popularization of RuO2 material, which almost fulfils all typical pseudocapacitor requirements.14,15 Metal oxides and conductive polymers are two main active electrode materials for pseudocapacitors that have been widely studied all over the world for a long time.16–20 Among metal oxide materials, NiO material is regarded as a promising candidate due to its rapid charging/discharging rate, high theoretical capacitance (ca. 2573 F g−1) and decent rate capability.21–24 However, the redox reactions may also result in instability during cycling especially when the active materials are coated on electrodes. Tremendous research on NiO has been carried out during recent years but its theoretical capacitance is rarely attained.25–28 On the other hand, conductive polymers have achieved rapid development for their flexibility and plasticity.19,29–30 However, when only conductive polymer is used as the active material in a supercapacitor, there are still some drawbacks remaining to be solved such as low charging speed and poor electron transport rate.19
In this study, we developed a simple, binder-free and environmentally friendly method to synthesize a high performance polyaniline (PANI)/NiO pseudocapacitor electrode that concentrates the advantages of both transition metal oxides and conductive polymers. With uniform pores and high electrical conductivity, a nickel foam was selected as the current collector to provide a large enough supporting area for loading active materials and an efficient pathway for ion diffusion. On the another hand, the nickel foam was also used as the nickel source for the in situ hydrothermal growth of NiO material. In this way, a steadier bonding between active material and current collector was established and the stability of this electrode could be substantially enhanced. PANI was deposited directly onto the electrode after the hydrothermal process and then enabled by the cyclic voltammetry (CV) process. This binder-free system could help the electrode materials fully engage in the electrochemical reactions by boosting the charge accumulation and ion transport processes. A considerable specific capacity of 2565 F g−1 (1 A g−1) was finally achieved, which was associated with the establishment of a fascinating nanostructure displaying the synergistic effect of the two active materials. A series of control experiments were carried out to identify the optimal synthesis conditions. Rate capability and cycle stability also proved that this PANI/NiO electrode had a wide practicability in developing high-quality supercapacitor devices.
Experimental
Materials and methods
This PANI/NiO electrode was synthesized using a hydrothermal method and in situ polymerization. All the chemical supplies were from Beijing Chemical Works including oxalic acid, hydrochloric acid, nickel foam, ammonium persulfate and aniline.Material characterization
The morphology of the PANI/NiO electrodes was characterized by a field-emission scanning electron microscope (FE-SEM, JSM-6700F, JEOL, Japan) at 5 kV. X-ray powder diffraction (XRD, SHIMADZU XRD-6000) was also carried out using Ni-filtered Cu-Kα radiation at 40 kV and 200 mA with 2θ ranging from 10° to 80° and a step scan rate of 5° min−1 in order to prove the existence of PANI.Electrochemical experiments were carried out in a three-electrode system on a CHI660D electrochemical workstation in which the PANI/NiO electrode and platinum sheet were used as working electrode and counter electrode, respectively. Hg/HgO electrode was used as the reference electrode and aqueous KOH solution (2 mol L−1, with O2 eliminated by charging with pure N2) was used as electrolyte. The cyclic voltammetry experiment was carried out in a potential range from 0 V to 0.65 V (vs. Hg/HgO) at varying scan rates of 2, 5, 10, 20, 50 and 100 mV s−1. Galvanostatic charge/discharge (GCD) properties were measured at step-increasing current densities of 1–10 A g−1 (potential range from 0 V to 0.55 V). Electrochemical impedance spectroscopy (EIS) was performed at open circuit potential, with 5 mV amplitude, and frequencies that ranged from 0.01 Hz to 100 kHz. The cycle life of the PANI/NiO electrode was measured by a battery testing system (Neware) with the cycle times of 5000.
Results and discussion
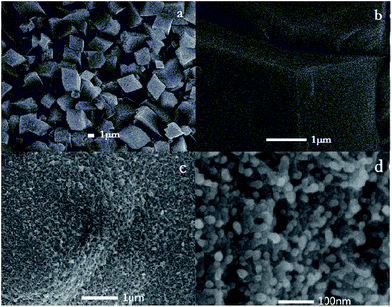
This PANI/NiO electrode was synthesized by depositing aniline directly onto the surface of a nickel oxalate electrode. It is the synergistic effect of these two active materials that largely enhances the energy storage performance. The specific capacity of the electrode nearly approached the theoretical value of a traditional NiO electrode. Importantly, the cycle stability of the composites was remarkable and all this is due to the uniform growth of polyaniline during the second-step.From the XRD measurements, a typical pattern of PANI/NiO composite (Fig. 1a) showed two distinct diffraction peaks at about 21° and 25°, which were considered with an amorphous form of polyaniline. The broad diffraction peaks can be ascribed to the poor crystallinity and thinness of the PANI coating. The NiO material was synthesized by an electrochemical oxidation process of nickel oxalate. This led to a nearly amorphous form of NiO observed through the XRD pattern that corresponds to (111), (220) and (222) peaks based on (PDF#71-1179). Because amorphous polyaniline provides a short ion diffusion pathway through the nickel oxide material, this composite could perform much better than the traditional NiO electrode. The N2 absorption–desorption isotherms and the corresponding pore size distribution plots of the synthesized PANI/NiO electrode are shown in Fig. 1b. A type III isotherm is indicated with the hysteresis loop starting from P/P0 = 0.2 revealing an H3 type. This proved the existence of structural pores. The SEM images of the PANI/NiO electrode (Fig. 1c and d) further confirmed this. As can be seen, flower-like nanostructures could be identified on the PANI/NiO electrode surface with the length of the ‘petals’ of about 100 nm to 8 μm and intersecting at right angles. These flower-like nanostructures substantially enlarged the electrochemically active specific surface area and also improved the porosity. Benefitting from these, the composite could store more charges as well as exchange charges faster.
 | ||
| Fig. 1 (a) XRD pattern of PANI/NiO composite (b) N2 absorption–desorption isotherms and (c, d) SEM images of PANI/NiO electrode. | ||
The SEM images of treated nickel foam current collectors without PANI polymerization are shown in Fig. 2a and b.27 It is clear that the blocky structures of NiO are non-porous and only deposited nanocrystals can be found on the surface. Low superficial area and the lack of pore structures were the main reasons why traditional NiO electrodes can hardly approach theoretical specific capacity. In the contrast experiment, oxalic acid was used as protonic acid for doping. As can be seen in Fig. 2c and d, the surface of electrode was covered with nanosphere clusters. The size of these nanospheres was about 30–40 nm and of a reasonably homogeneous size distribution. Compared with hydrochloric acid doping, this 3D structure is more compact and provides a smaller active specific surface area. The active specific surface area could be used to gather active materials from solution and to some degree, decide the maximum faradic current density. In this connection, the PANI/NiO electrode with flower-like nanostructures might exhibit a better performance in rate capability.
In Fig. 3, the CV curves of the composite were studied over a potential range from 0 V to 0.65 V. The PANI/NiO electrode exhibited a pseudocapacitance characteristic with only one pair of obvious redox peaks at 0.43 V and 0.3 V. This was supposed to stem from the combined contributions from both NiO and PANI. NiO material played a major role in providing high specific capacitance due to the fast redox reactions. At the same time, the PANI material, covering the surface of NiO material, acted in a supporting role by optimizing pore structures and boosting mass transfer processes, which help the redox reactions of NiO proceed more quickly and more completely. Furthermore, the PANI material could shape the surface of the NiO electrode and elaborately bring in porous structures over it. With this help, the PANI/NiO material showed a superior electrochemical reversibility in comparison with the pure NiO electrode and a better capacity than pure PANI. In other words, the PANI material might not store charges on its own but improved the capability of the NiO material. Each component synergistically serves a specific purpose. Because of these synergistic effects, this composite can offer many more advantages than one pure electrode material. From the GCD curves, the specific capacitance could be calculated and reached a maximum of 2565.45 F g−1 at the current density of 1 A g−1. The rate capability was also calculated (Fig. 3c). As shown, the specific capacity still remained at about 71% (1818.19 F g−1) at the current density of 10 A g−1. From the EIS curves (inset of Fig. 3c), both the two electrodes exhibited a fairly low internal resistance as revealed by the intersections of the z′-axis and the high frequency part of the curves. The sharp vertical line at low frequency manifested an ideal capacitance characteristic of PANI/NiO electrode. This is more valuable in supercapacitor assembly compared with the NOe material. The impressive specific capacitance and decent rate capability demonstrated the great potential of the PANI/NiO material in a daily-use supercapacitor device considering its simple preparation technology.
To determine the best growth condition, a series of control experiments were carried out. Fig. 4 shows the CV curves at a scan rate of 5 mV s−1 and GCD curves of PANI/NiO materials synthesized at different pHs (range from 1 to 6) adjusted by either hydrochloric acid or oxalic acid. In comparison with those synthesized in oxalic acid, the composite synthesized in hydrochloric acid achieved a higher peak current and larger cover area at the same pH. This could be attributed to the larger surface area of the flower-like nanostructures that was more available for ion diffusion than that of the nanospheres. This result was completely in agreement with analysis of the SEM images. On the other hand, the anions might also contribute to the doping process during which the Cl− anion could form a charge transfer complex more easily. In terms of the CV curves of hydrochloric acid-doped composite, no rigorous linear relationship between pH and specific capacitance could be identified. Only a best performance condition was distinguished when the pH was 3. Based on the galvanostatic discharge curves studied at 1 A g−1, when using hydrochloric acid, the specific capacity of the PANI/NiO electrode was determined to be 495 F g−1, 323 F g−1, 2565 F g−1, 878 F g−1, 383.45 F g−1, and 495 F g−1 for pH 1, 2, 3, 4, 5 and 6, respectively. When using oxalic acid, the specific capacity was 810 F g−1, 1478 F g−1, 1515 F g−1, 1625 F g−1, 2501 F g−1 and 1830 F g−1 for pH 1, 2, 3, 4, 5 and 6, respectively. The highest specific capacity (2565 F g−1) nearly approached the theoretical specific capacitance of the NiO material. This should be due to the synergistic effect of these two electrode materials that grew stepwise on nickel foam. Thus, the best growing conditions can be concluded to be as follows: the pH is 3 and hydrochloric acid is used for doping, when the PANI/NiO electrode could achieve a maximum specific capacitance.
Although a high capacitance has been achieved, the cycle stability was always a serious challenge to conductive polymers like PANI considering that the functional space structure could be destroyed during rapid charging and discharging processes at short intervals. The cycle life and energy efficiency of the PANI/NiO electrode are shown in Fig. 5. The energy efficiency remained at ∼60% during the first 200 cycles and then slowly decreased. Finally, it reached about 20%. In view of the cycle life, it was amazing that the retention ratio maintained a high value of 100% for almost all 5000 cycles. This outstanding performance was supposed to benefit from the strong bonding through in situ polymerization. This impressive cycling stability shows a considerable potential for commonly used energy storage devices with long service life.
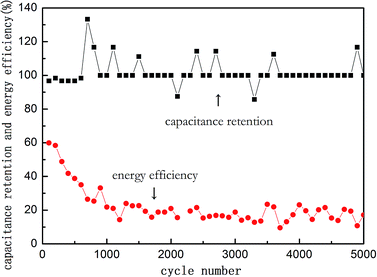 | ||
| Fig. 5 Capacitance retention and energy efficiency curves of PANI/NiO electrode produced in best condition. | ||
Conclusions
In summary, a facile, simple, two-step, in situ growth method was developed and systematized to synthesize a high specific capacity PANI/NiO composite material on nickel foam as a supercapacitor electrode. The impressive performance of this electrode was demonstrated and analysed in the results. Benefitting from the flower-like nanostructures and the synergistic contributions from both NiO and PANI, a porous electrode surface with high specific surface area was presented as the basis for a considerable specific capacitance. When using hydrochloric acid for doping, the PANI/NiO electrode exhibited a maximum specific capacitance of 2565 F g−1 at 1 A g−1. In addition, the cycle stability also showed decent performance during 5000 cycles. All the abovementioned results demonstrate the potential of this composite in terms of its application in supercapacitors where high energy density and long term stability are required.Acknowledgements
This work is financially supported by the State Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University and the National Natural Science Foundation of China (Grant No. 61474056 and No. 61374218).Notes and references
- J. Zhang and X. S. Zhao, ChemSusChem, 2012, 5, 818–841 CrossRef CAS PubMed.
- J. Yan, Q. Wang, T. Wei and Z. Fan, Adv. Energy Mater., 2014, 4, 1300816 Search PubMed.
- C. Zhou, Y. Zhang, Y. Li and J. Liu, Nano Lett., 2013, 13, 2078–2085 CrossRef CAS PubMed.
- C. Yuan, J. Li, L. Hou, X. Zhang, L. Shen and X. W. D. Lou, Adv. Funct. Mater., 2012, 22, 4592–4597 CrossRef CAS.
- X. Zhao, B. M. Sanchez, P. J. Dobson and P. S. Grant, Nanoscale, 2011, 3, 839–855 RSC.
- A. Burke, Ultracapacitors why, how, and where is the technology, Elsevier Science, 2000 Search PubMed.
- M.-S. Park, S. Cho, E. Jeong and Y.-S. Lee, J. Ind. Eng. Chem., 2015, 23, 27–32 CrossRef CAS.
- C. Wolff, S. Jeong, E. Paillard, A. Balducci and S. Passerini, J. Power Sources, 2015, 293, 65–70 CrossRef CAS.
- K. H. Teoh, C.-S. Lim, C.-W. Liew, S. Ramesh and S. Ramesh, Ionics, 2015, 21, 2061–2068 CrossRef CAS.
- A. Jain and S. K. Tripathi, Ionics, 2014, 21, 1391–1398 CrossRef.
- S. Majumder, S. Dey, K. Bagani, S. K. Dey, S. Banerjee and S. Kumar, Dalton Trans., 2015, 44, 7190–7202 RSC.
- Y. Gogotsi and P. Simon, Science, 2011, 334, 917–918 CrossRef CAS PubMed.
- G. He, J. Li, W. Li, B. Li, N. Noor, K. Xu, J. Hu and I. P. Parkin, J. Mater. Chem. A, 2015, 3, 14272–14278 CAS.
- M. T. Brumbach, T. M. Alam, R. H. Nilson, P. G. Kotula, B. B. McKenzie, R. G. Tissot and B. C. Bunker, Mater. Chem. Phys., 2010, 124, 359–370 CrossRef CAS.
- J.-Y. Kim, K.-H. Kim, S.-H. Park and K.-B. Kim, Electrochim. Acta, 2010, 55, 8056–8061 CrossRef CAS.
- M. Huang, F. Li, F. Dong, Y. X. Zhang and L. L. Zhang, J. Mater. Chem. A, 2015, 3, 21380–21423 CAS.
- X. Sun, W. Si, X. Liu, J. Deng, L. Xi, L. Liu, C. Yan and O. G. Schmidt, Nano Energy, 2014, 9, 168–175 CrossRef CAS.
- S. Faraji and F. N. Ani, J. Power Sources, 2014, 263, 338–360 CrossRef CAS.
- I. E. Rauda, V. Augustyn and B. Dunn, Acc. Chem. Res., 2013, 46, 1113–1124 CrossRef CAS PubMed.
- W. Ji, J. Ji, X. Cui, J. Chen, D. Liu, H. Deng and Q. Fu, Chem. Commun., 2015, 51, 7669–7672 RSC.
- M. Huang, F. Li, J. Y. Ji, Y. X. Zhang, X. L. Zhao and X. Gao, CrystEngComm, 2014, 16, 2878 RSC.
- L. Feng, Y. Zhu, H. Ding and C. Ni, J. Power Sources, 2014, 267, 430–444 CrossRef CAS.
- N. Padmanathan, S. Selladurai, K. M. Rahulan, C. O'Dwyer and K. M. Razeeb, Ionics, 2015, 21, 2623–2631 CrossRef CAS.
- K. Tao, P. Li, L. Kang, X. Li, Q. Zhou, L. Dong and W. Liang, J. Power Sources, 2015, 293, 23–32 CrossRef CAS.
- X. Sun, C. Yan, Y. Chen, W. Si, J. Deng, S. Oswald, L. Liu and O. G. Schmidt, Adv. Energy Mater., 2014, 4, 1300912 Search PubMed.
- Y. Jiang, Z. Jia, W. Zhang and H. Suo, J. Inorg. Organomet. Polym. Mater., 2013, 23, 1043–1047 CrossRef CAS.
- Y. Jiang, X. Leng, Z. Jia, H. Chen, H. Suo and C. Zhao, J. Mater. Sci.: Mater. Electron., 2015, 26, 2995–3000 CrossRef CAS.
- S. Lv, C. Wang and S. Xing, J. Alloys Compd., 2014, 603, 190–196 CrossRef CAS.
- X. Li, X. Liu, X. Qiao and S. Xing, RSC Adv., 2015, 5, 79172–79177 RSC.
- G. A. Snook, P. Kao and A. S. Best, J. Power Sources, 2011, 196, 1–12 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |