Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Use of a novel layered titanoniobate as an anode material for long cycle life sodium ion batteries†
Keyu
Xie
a,
Wenfei
Wei
a,
Haoran
Yu
a,
Manjiao
Deng
b,
Shanming
Ke
*b,
Xierong
Zeng
b,
Zhihua
Li
c,
Chao
Shen
a,
Jian-gan
Wang
a and
Bingqing
Wei
*ad
aState Key Laboratory of Solidification Processing and Center for Nano Energy Materials, Northwestern Polytechnical University, Xi'an, 710072, China. E-mail: weib@udel.edu
bShenzhen Key Laboratory of Special Functional Materials, College of Materials Science and Engineering, Shenzhen University, Shenzhen 518060, PR China. E-mail: smke@szu.edu.cn
cSchool of Materials Science and Engineering, Central South University, Changsha, Hunan 410083, China
dDepartment of Mechanical Engineering, University of Delaware, Newark, DE 19716, USA
First published on 30th March 2016
Abstract
Sodium ion batteries have been attracting increasing attention as a replacement for lithium ion batteries in large-scale energy applications. However, it is proving difficult to find suitable sodium host materials with both a high capacity and excellent cycle stability. We prepared a layered titanoniobate (HTi2NbO7) via solid-state calcination followed by ion exchange for use in sodium ion batteries. The lamellar HTi2NbO7 had a specific capacity of about 90 mA h g−1 at 100 mA g−1. The capacity was highly reversible over 2000 cycles. These results show that this lamellar titanoniobate material is a promising anode material for sodium ion batteries with a long cycle life.
Sodium ion batteries have been attracting much attention as a replacement for lithium ion batteries as a result of the low cost and high abundance of sodium resources.1 When used in large-scale energy storage systems, where cost and sustainability are major concerns, the superiority of sodium ion batteries over their lithium ion counterparts becomes important.2,3 However, it is difficult to find a suitable electrode material for sodium ion batteries as a result of the large ionic radius of sodium (70% larger than that of lithium).
A number of transition metal oxides show promise for electrochemical energy storage.4 Titanium- and niobium-based oxides have been widely investigated as anode materials for lithium ion batteries as a result of the beneficial redox potentials of Nb5+/Nb4+, Ti4+/Ti3+ and Nb4+/Nb3+.5–7 A mixed titanium–niobium oxide (TiNb2O7) used as a novel anode for lithium ion batteries had a high theoretical capacity of up to 387.6 mA h g−1.8 This mixed titanium–niobium oxide has all the above-mentioned redox couples (Nb5+/Nb4+, Ti4+/Ti3+ and Nb4+/Nb3+). As a result, a high specific capacity is expected because five lithium ions can theoretically be inserted into one formula unit of TiNb2O7. Other titanium–niobium oxide based materials, such as Ti2Nb2O9,9 TiNb2O7,10,11 TiNb6O17 (ref. 12) and Ti2Nb10O29,13 have also been studied as electrode materials for lithium ion batteries and have shown excellent performance. Surprisingly, the family of titanium–niobium oxide based electrode materials also shows excellent insertion/extraction of lithium. Ti2Nb10O29 has a reversible specific capacity of 238 mA h g−1 at 2C (1C = 396 mA g−1) after 800 cycles.13 However, as far as we know, there has been no report of the electrochemical properties of the mixed titanium–niobium oxide with multiple redox couples (Nb5+/Nb4+, Ti4+/Ti3+ and Nb4+/Nb3+) for sodium intercalation.
In a similar manner, Na2Ti3O7, which is also a member of the AxM2nO4n+2 family of layered titanoniobates, should provide the possibility of lithium/sodium intercalation.14 There have been few reports on sodium intercalation in these kinds of materials, which have both the easily reducible species Ti4+ and Nb5+ and a lamellar structure.15 We report here the synthesis of the layered titanoniobate HTi2NbO7via solid-state calcination followed by ion exchange and the first experimental results of the electrochemical properties of HTi2NbO7 as an anode material for sodium ion batteries. The preliminary results showed that HTi2NbO7 had a reversible specific capacity of about 90 mA h g−1 at 100 mA g−1 for sodium ion intercalation/de-intercalation after 2000 cycles.
Scheme 1 shows the manufacturing process for HTi2NbO7·H2O. TiO2, Nb2O5 and Cs2CO3 (a 10% molar excess) were first ground in a mortar for about 30 min and then heated at 1373 K for 12 h. A proton exchange reaction was then carried out by adding CsTi2NbO7 to an acidic solution (1 M) with magnetic stirring at 333 K for 24 h. The precipitate was filtered and washed three times with deionized water. HTi2NbO7 was obtained by heating the precipitate under vacuum at 423 K for 2 h.
Fig. 1a and b shows the X-ray diffraction patterns of CsTi2NbO7 and HTi2NbO7, which match well with the orthorhombic crystallographic system;16 all the diffraction peaks were in accordance with those of JCPDS Card no. 73-0680 and JCPDS Card no. 54-1154, respectively. These results show that there was no impurity in the products and the interlamellar spacing was reduced.17Fig. 1c shows the structure of HTi2NbO7. The H+ structure is built up from layers containing zigzag strings with groups of three edge-shared octahedra. The strings are linked side by side via corner sharing to form Ti2NbO7− layers. The H+ ions occupy distorted cubic sites between the layers. Two adjacent layers are related by a glide plane in both structures.17
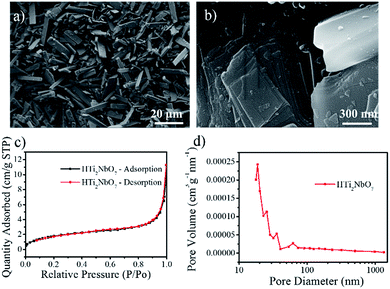
The morphology of the cuboid arrangement is shown in Fig. 2a, the scanning electron microscopy (SEM) image, which shows a uniform width distribution of ∼2 μm. Fig. 2b clearly shows that the lamellar titanoniobate HTi2NbO7 has a layered structure. The nitrogen adsorption/desorption isotherm and pore size distribution plots show the characteristics of this mesoporous material (Fig. 2c and d). The material has a relatively low BET surface area of 6.7 m2 g−1 and the pore size distribution is mainly from 2 to 3 nm. The mesoporous laminated structure could give a greater active area for Na+.
XPS was used to investigate the surface composition and valence states of HTi2NbO7. Photoelectron peaks for C, O, Ti and Nb can been clearly seen in Fig. 3a. Fig. 3b confirmed the existence of the Ti4+ oxidation state from the Ti 2p1/2 and 2p3/2 peaks at 464.2 and 458.3 eV with a spin–orbit splitting of about 5.9 eV.18Fig. 3c shows the Nb 3d spectrum for HTi2NbO7, with two peaks at 206.8 and 209.5 eV. These two peaks represent the 3d5/2 and 3d3/2 components, respectively, with a spin–orbit splitting of 2.7 eV. The center of the Nb 3d3/2 peak corresponds to the Nb5+ oxidation state.19
 | ||
| Fig. 3 (a) XPS survey spectra for the surfaces of the HTi2NbO7 sample. Typical spectra of (b) Ti 2p and (c) Nb 3d. | ||
The electrochemical performance of the layered HTi2NbO7 anode were studied by galvanostatic charge–discharge measurements. Each cell was tested at potentials within the range 0.01–3 V. Fig. 4a shows the initial charge–discharge voltage profiles at 100 mA g−1. The discharge curves show four different regions: two sloping regions at 1.5–1.25 and 0.98–0.86 V; a plateau at 0.45 V; and a further plateau region up to the discharge limit. The voltage decreased sharply to 1.5 V, mainly due to a solid solution reaction. The two sloping regions at 1.5–1.25 and 0.98–0.86 V and the plateau at 0.45 V are attributed to a two-phase reaction for Nb5+/Nb4+, Ti4+/Ti3+ and Nb4+/Nb3+, respectively.20 There is another long plateau from 0.16 to 0.01 V, which may correspond to the process in which H+ ions are simultaneously replaced by Na+ ions during the electrochemical intercalation.15 During the first discharge, the specific capacity reaches 430 mA h g−1 with a reversible capacity of about 200 mA h g−1. The irreversible capacity can be mainly attributed to the transformation of the structure resulting from the irreversible process of Na+ replacing H+ and the formation of the solid electrolyte interface (SEI).15 From the tenth cycle, the capacity gradually become stable, showing that the structure tended to become steady after all the H+ had been replaced by Na+.
Cyclic voltammetry curves of the HTi2NbO7 anode at a scanning rate of 0.01 mV s−1 over the voltage range 0.01–3.0 V are shown in Fig. 4b. There are two small peaks around 1.5 and 0.98 V as a result of the valence variation of Nb5+/Nb4+ and Ti4+/Ti3+; a sharp peak at 0.45 V could correspond to the Nb4+/Nb3+ redox couple.20 The last sharp sloping peak at 0.01 V may be due to the replacement of H+ by Na+. The CV curves are in agreement with the charge–discharge curves.
Long-term cycle stability is a challenge for practical sodium ion batteries as a result of the possible structural degradation of the host during cycling. Fig. 4c shows the superior cycling performance of the HTi2NbO7 electrode at 100 mA g−1. Although a slow fading of capacity is seem in the initial few dozen cycles, there was no decline in capacity during the subsequent 2000 cycles. In addition, a reversible capacity of about 90 mA h g−1 remained unchanged during the subsequent 2000 cycles, indicating excellent cycle stability. With respect to the contribution of conductive carbon, the capacity of pure HTi2NbO7 is about 70 mA h g−1 (ESI, Fig. S1†). Notably, the layered structure of the HTi2NbO7 was almost unchanged after 2000 cycles, although it was covered with a thick SEI film (ESI, Fig. S2†). Correspondingly, the coulombic efficiency gradually increased during the initial cycles and remained at >99% in the following cycles. The performance of this HTi2NbO7 electrode is better than that of other materials reported for sodium ion batteries, such as Nb2O5 (about 30 mA h g−1 after 50 cycles at 50 mA g−1),21 K0.8Ti1.73Li0.27O4 (about 80 mA h g−1 at 100 mA g−1),22 Na2/3Co1/3Ti2/3O2 (50 mA h g−1 at 500 mA g−1),23 TiO2 nanoparticles (93 mA h g−1 at 1700 mA g−1),24 Na2Ti6O13 (about 100 mA h g−1 after 30 cycles at 5 mA g−1)25 and Cu0.5TiOPO4 (70 mA h g−1 after 20 cycles at 42.2 mA g−1).26
To further understand the electrochemical characteristics of HTi2NbO7, rate capability tests were performed at different current densities (Fig. 4d). A reversible capacity of 106 mA h g−1 was obtained at a low current density of 40 mA g−1. When the current density increased to 100 and 200 mA g−1, reversible specific capacities of 77 and 62 mA h g−1 were obtained respectively. Even though the current density increased to 1000 mA g−1, a capacity of about 32 mA h g−1 was still maintained. When the current density reversed back to 40 mA g−1, the capacity of the HTi2NbO7 still remained at about 85 mA h g−1. Taking into account the contribution of conductive carbon, the capacity of pure HTi2NbO7 is reduced by a certain ratio. These results indicate that HTi2NbO7 has a good rate performance.
EIS was also carried out to obtain insights into the electrochemical behavior of HTi2NbO7. These data were recorded before cycling and after 100 cycles (Fig. 5). As Fig. 5a shows, the Nyquist plots consist of one semicircle at high frequencies and a straight line at low frequencies. Fig. 5b shows that the Nyquist plots consist of two anomalous semicircles at high frequencies after 100 cycles at 100 mA h g−1. The equivalent circuit was used to determine the resistance of the cells after 100 cycles in detail (Fig. 5c). Table 1 shows the fitted EIS values of the equivalent circuit elements. The intercept at the real axis at high frequency gives the electrolyte resistance (Rs) for the battery.27 The semicircle is usually attributed to the SEI film (Rsei and Csei) and the charge transfer process through the electrode surface (Rct and CPE(Cdl)), respectively.28 The straight line in the low frequency domain is the Warburg impedance (W), which is attributed to the diffusion of Na ions in the electrode.29 There was no SEI film before cycling; after 100 cycles the radius of the semicircle at medium frequencies was smaller than that of the battery before cycling. The decrease in Rct is attributed to the formation of a SEI film on the cycled electrode, which enhances the reaction kinetics, as reported previously.30,31 The Warburg impedance of the battery after 100 cycles was much lower than that of the battery before cycling, which indicates that the polarization of the HTi2NbO7 anode decreased dramatically favors capacity retention during cycling.32
 | ||
| Fig. 5 Impedance plots of HTi2NbO7 for (a) before cycling and (b) after 100 cycles at 100 mA h g−1. (c) Equivalent circuit for simulating the EIS data about the battery after 100 cycles. | ||
| Parameter | Value |
|---|---|
| R s (Ω) | 11.18 |
| C sei | 1.3091 × 10−6 |
| R sei (Ω) | 5.364 |
| CPE-T(Cdl) | 1.8005 × 10−5 |
| CPE-P(Cdl) | 0.89822 |
| R ct | 77.67 |
| W o-R | 23.53 |
| W o-T | 0.017247 |
| W o-P | 0.40037 |
We have successfully fabricated a layered HTi2NbO7 structure via a solid-state reaction followed by ion exchange and used it as an anode material in sodium ion batteries. Electrochemical tests indicated that this layered material can give a reversible capacity of about 90 mA h g−1 with no capacity fading after 2000 cycles in the voltage range 0.01–3.0 V. This is an exciting option for the synthesis and design of new materials with layered structures as high-performance anodes for sodium ion batteries. The material displayed excellent cycle performance and we believe that these results represent a significant step forward in the development of long-life sodium ion batteries for large-scale applications in novel energy storage devices.
Acknowledgements
The authors appreciate financial support from the National Natural Science Foundation of China (No. 51302219, 51472204, 51402236 and 51302172), the Shenzhen Key Laboratory of Special Functional Materials (T201504), the Natural Science Foundation of Shannxi Province (No. 2015JM2045), the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20136102120024), the Research Fund of the State Key Laboratory of Solidification Processing (NWPU), China (Grant No. 06-QP-2014) and the Fundamental Research Funds for the Central Universities (No. 3102014JCQ01019).Notes and references
- V. Palomares, P. Serras, I. Villaluenga, K. B. Hueso, J. Carretero-González and T. Rojo, Energy Environ. Sci., 2012, 5, 5884–5901 CAS.
- S.-W. Kim, D.-H. Seo, X. Ma, G. Ceder and K. Kang, Adv. Energy Mater., 2012, 2, 710–721 CrossRef CAS.
- M. D. Slater, D. Kim, E. Lee and C. S. Johnson, Adv. Funct. Mater., 2013, 23, 947–958 CrossRef CAS.
- J. Zhang and A. Yu, Sci. Bull., 2015, 60, 823–838 CrossRef CAS.
- M. A. Reddy and U. V. Varadaraju, Chem. Mater., 2008, 20, 4557–4559 CrossRef CAS.
- S.-H. Kang, D. P. Abraham, W.-S. Yoon, K.-W. Nam and X.-Q. Yang, Electrochim. Acta, 2008, 54, 684–689 CrossRef CAS.
- J.-T. Han, D.-Q. Liu, S.-H. Song, Y. Kim and J. B. Goodenough, Chem. Mater., 2009, 21, 4753–4755 CrossRef CAS.
- J.-T. Han, Y.-H. Huang and J. B. Goodenough, Chem. Mater., 2011, 23, 2027–2029 CrossRef CAS.
- J.-F. Colin, V. Pralong, M. Hervieu, V. Caignaert and B. Raveau, Chem. Mater., 2008, 20, 1534–1540 CrossRef CAS.
- C. Jo, Y. Kim, J. Hwang, J. Shim, J. Chun and J. Lee, Chem. Mater., 2014, 26, 3508–3514 CrossRef CAS.
- B. Guo, X. Yu, X.-G. Sun, M. Chi, Z.-A. Qiao, J. Liu, Y.-S. Hu, X.-Q. Yang, J. B. Goodenough and S. Dai, Energy Environ. Sci., 2014, 7, 2220–2226 CAS.
- C. Lin, G. Wang, S. Lin, J. Li and L. Lu, Chem. Commun., 2015, 51, 8970–8973 RSC.
- Q. Cheng, J. Liang, Y. Zhu, L. Si, C. Guo and Y. Qian, J. Mater. Chem. A, 2014, 2, 17258–17262 CAS.
- J.-F. Colin, V. Pralong, V. Caignaert, M. Hervieu and B. Raveau, Inorg. Chem., 2006, 45, 7217–7223 CrossRef CAS PubMed.
- J. F. Colin, V. Pralong, M. Hervieu, V. Caignaert and B. Raveau, J. Mater. Chem., 2008, 18, 3121 RSC.
- M. Hervieu and B. Raveau, J. Solid State Chem., 1980, 32, 161–165 CrossRef CAS.
- M. Lal and A. T. Howe, J. Mater. Chem., 1984, 51, 355–363 CrossRef CAS.
- C. Ban, M. Xie, X. Sun, J. J. Travis, G. Wang, H. Sun, A. C. Dillon, J. Lian and S. M. George, Nanotechnology, 2013, 24, 424002 CrossRef PubMed.
- M. Z. Atashbar, H. T. Sun, B. Gongc, W. Wlodarski and R. Lamb, Thin Solid Films, 1998, 326, 238–244 CrossRef CAS.
- V. Aravindan, J. Sundaramurthy, A. Jain, P. S. Kumar, W. C. Ling, S. Ramakrishna, M. P. Srinivasan and S. Madhavi, ChemSusChem, 2014, 7, 1858–1863 CrossRef CAS PubMed.
- H. Kim, E. Lim, C. Jo, G. Yoon, J. Hwang, S. Jeong, J. Lee and K. Kang, Nano Energy, 2015, 16, 62–70 CrossRef CAS.
- K. Y. Chen, W. X. Zhang, Y. Liu, H. P. Zhu, J. Duan, X. H. Xiang, L. H. Xue and Y. H. Huang, Chem. Commun., 2015, 51, 1608–1611 RSC.
- H. Yu, Y. Ren, D. Xiao, S. Guo, Y. Zhu, Y. Qian, L. Gu and H. Zhou, Angew. Chem., Int. Ed., 2014, 53, 8963–8969 CrossRef CAS PubMed.
- Z. Hong, K. Zhou, Z. Huang and M. Wei, Sci. Rep., 2015, 5, 11960 CrossRef PubMed.
- K. Shen and M. Wagemaker, Inorg. Chem., 2014, 53, 8250–8256 CrossRef CAS PubMed.
- P. Bleith, H. Kaiser, P. Novák and C. Villevieille, Electrochim. Acta, 2015, 176, 18–21 CrossRef CAS.
- S. A. Needham, G. X. Wang, K. Konstantinov, Y. Tournayre, Z. Lao and H. K. Liu, Electrochem. Solid-State Lett., 2006, 9, A315 CrossRef CAS.
- S. S. Zhang, K. Xu and T. R. Jow, Electrochim. Acta, 2004, 49, 1057–1061 CrossRef CAS.
- K. Tang, L. Fu, R. J. White, L. Yu, M.-M. Titirici, M. Antonietti and J. Maier, Adv. Energy Mater., 2012, 2, 873–877 CrossRef CAS.
- K. Y. Xie, Z. G. Lu, H. T. Huang, W. Lu, Y. Q. Lai, J. Li, L. M. Zhou and Y. X. Liu, J. Mater. Chem., 2012, 22, 5560–5567 RSC.
- Y. Liu, C. H. Mi, L. H. Su and X. G. Zhang, Electrochim. Acta, 2008, 53, 2507–2513 CrossRef CAS.
- N. V. Nghia, P.-W. Ou and I. M. Hung, Ceram. Int., 2015, 41, 10199–10207 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/c6ra02530g |
| This journal is © The Royal Society of Chemistry 2016 |