Increasing cell viability using Cd-free – InP/ZnS@silica@layered double hydroxide – materials for biological labeling†
Iván Castelló
Serrano
*ab,
Georgiana
Stoica
a and
Emilio
Palomares
ac
aInstitute of Chemical Research of Catalonia (ICIQ), Avinguda del Països Catalans 16, 43007 Tarragona, Spain
bTelethon Institute of Genetics and Medicine (TIGEM), Campi Flegrei 34, 80078 Pozzuoli, Naples, Italy. E-mail: i.castello@tigem.it; Tel: +39-081-19230600
cInstitució Catalana de Recerca I Estudis Avançats (ICREA), Passeig Lluís Companys 23, E-08010 Barcelona, Spain
First published on 22nd March 2016
Abstract
In this work we describe the synthesis and characterization of InP/ZnS@silica@LDH nanoparticles and, moreover, their use as biomarkers. The use of dual systems combining silica shells and hydrotalcite nanocoatings favors the cell viability in clear contrast with the system with only silica shells. Furthermore, the use of Cd-free luminophores extends the cells lifetime and illustrates the potential of the InP/ZnS@silica@LDH material as a biomarker.
Introduction
The use of nanoparticles for biological labeling, drug delivery, and cell apoptosis among other applications is of increasing interest in the field of bio-nanoscience. In all cases, the first step that determines the successful application of the material is the cell uptake process. Although the introduction of the material inside the cell can be carried out in an “active” way, which involves energy to go against some gradient or impediment, in some cases the cellular uptake takes place through the adsorption of the material in a “passive” way, which depends on the cell membrane permeability, which in turn depends on the membrane composition (proteins and lipids). However, the latter case requires that the material size is in a few nanometers range.1–4Quantum dots have been extensively used as biological markers due to their small nanometer size (2–5 nm) and their remarkable luminescence properties that are highly tunable due to the quantum nature of the materials as, for example, CdSe or CdTe. However, if the experiment to carry out requires good cell viability, it is desirable to avoid the use of toxic materials including lead, cadmium and tellurium.5
As an alternative, heavy metal-free QDs such as InP/ZnS quantum dots,6 carbon dots7,8 and graphene quantum dots9 have attracted much attention. Besides, embedding the QDs into silica shells results appropriated in view of minimizing the impact of the materials composition leaching in the biological media, in particular for bio-labelling and/or drug delivery.10 Pioneering studies started using silica to coat QDs, leading to applications in many fields, with particular emphasis on molecules carrier and drug delivery systems.11 The delivery of molecules inside mammalian cells with the aim of transferring them across the cell membrane into the cytoplasm is a research area of constantly increasing importance in medicine. Direct delivery is generally inefficient and suffers from problems such as enzymatic degradation, poor bioavailability, poor stability, undesirable accumulative effects of the carrier, and many others.12 However, a considerable number of studies have been published throughout the last years,13,14 concluding that the uptake and cytotoxicity of silica nanoparticles (NPs) is size and cell-type dependent, involving either clathrin- or caveolin-mediated endocytosis.15
In order to benefit at maximum of the opportunities offered by the silica NPs in the biomedical fields, it is evident that these particles need to be highly protected, yet displaying excellent cellular uptake and transport features. In the present study, we tackled this aspect by coating the InP/ZnS@silica core shell NPs with layered double hydroxides (LDHs). In the recent years, it has become apparent that LDHs, also known as hydrotalcites (HTs) are an excellent carrier material due to their outstanding properties with respect to the cell membrane transport.12 Although the intercalation of molecules by anionic exchange or delamination-restacking decreases their usually positive surface charge, they remain satisfactorily positively charged to facilitate the cellular uptake.16–19 The HT can be used as host matrix for drugs, luminescent dyes but also for nanoparticles (QDs, QD@silica nanospheres, silica NPs, nanorods and lanthanides), enhancing their photoluminescence and stability.11,20–26
To the best of our knowledge, hydrotalcite-like materials encapsulating luminescent Cd-free QDs@silica nanospheres were employed herein for the first time for cell imaging. Moreover, we further explored the influence of LDHs on the cellular uptake and cell cytotoxicity for the HK-2 cell line, by restricting the optimal nanoparticle size.
Results and discussion
In a previous study,19 we demonstrated that quantum dots release from the solid structure quantum dot-LDH. In the very same work we also reported that Cd-based QDs luminescence is really sensitive to the cations and anions in the environment. The same holds for InP/ZnS QDs.27 In order to prevent the escape of quantum dots we used tailor-made LDH as protecting shells for quantum dots embedded into silica nanospheres without changing either the materials or the optical properties. Furthermore, the silica layer confers protection to the QDs surface and avoids any change by surrounding molecules, such as cations, anions or biomolecules that could affect their emission properties.28The InP/ZnS@silica nanospheres (Fig. 1A) have been synthesized using previously reported methods.10,26 Thereafter, the nanospheres (17–65 nm) were coated with LDH nanosheets (Fig. 1B) leading to QD@silica@LDH nanoparticles with a final diameter from 22 to 70 nm (please see the ESI, Fig. S1†). A uniform layer of approximately 2.5–3 nm LDH nanosheets was deposited on the surface of the QD@silica nanospheres, as indicated by the electron microscopy images Fig. 1.
 | ||
| Fig. 1 Typical TEM images of 55 nm original InP/ZnS@silica nanospheres (A) and 60 nm resulting QD@silica@LDH hybrid nanocomposites (B), respectively. | ||
These observations are in good agreement with atomic force microscopy results,29,30 which revealed the existence of LDH nanosheets of an average height profile 1.2–2 nm. Considering this, we can assume the LDH layer on the surface of QD@silica nanospheres corresponds to only a few (two to three) unilamellar nanosheets.
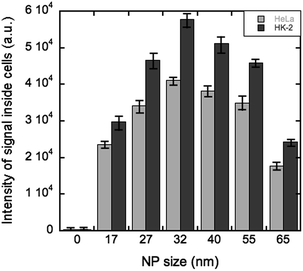
Fig. 2 shows the representative confocal images for the HK-2 cells incubated with the QD@silica@LDH nanoparticles. On the one hand, the nanoparticles with diameters between 32 nm and 40 nm showed higher cell uptake. On the other hand, larger spheres with diameters between 55 nm and 65 nm were introduced in the cells in a lesser extent. Interestingly, smaller nanoparticles (17 nm and 27 nm) also displayed a lower uptake by the HK-2 cells.
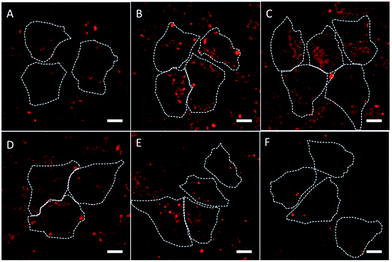 | ||
| Fig. 2 Confocal images of HK-2 cells incubated at 37 °C with 15 nM of QD@silica@LDH NPs for 30 min: (a) 17 nm, (b) 27 nm, (c) 32 nm, (d) 40 nm, (e) 55 nm, and (f) 65 nm. The red fluorescence, arising from the InP/ZnS inside the NPs (dashed lines), was collected in the range 610–690 nm after excitation at 405 nm (Fig. S2†). The scale bars correspond to 5 µm. | ||
Fig. 3 shows the cell culture luminescence intensity versus the nanoparticle size. As it can be seen, the luminescence is higher for nanoparticles with 32 nm diameter, in good agreement with the higher cell uptake of these particles for the HK-2 cells (Z-stack movies in ESI†). The HeLa cells were used as control as it is known that these particular type of cells is more robust (less sensitive) towards the use of silica coating nanoparticles. The control and the HK-2 cells show similar behavior which implies that the hydrotalcite coated nanoparticles do not affect different type of cell lines with different sensitivity towards silica coated nanoparticles.
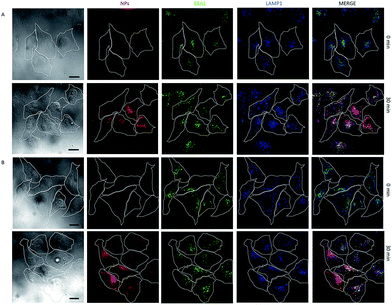
Our results are in line with previously reported experimental studies on targeted drug delivery into cells and uptake of nanoparticles, which have identified the particle size as an important factor in the cellular uptake. Nonetheless, Mou et al.31 found that silica nanospheres of 50 nm in diameter were the most suitable candidate for the cellular uptake of nanoparticles. However, in case of nanoparticles smaller than 10 nm in diameter, the nanoparticles are accumulated on the cell membrane prior to internalization as reported by Treuel et al.32 From our experiments however, we observed that silica nanospheres of 32 nm are optimal in the case of the HK-2 cell line. Our hypothesis is that the use of the LDH coating improves the cellular uptake. In fact, our observations using confocal microscopy suggest that for the QD@silica@LDH nanoparticles of 32 nm, after 30 min of incubation, the material is located in the endosomes and cell cytosol (Fig. 4), which is a consequence of the LDH shell onto the QD@silica NPs. Xu et al.33 confirmed that LDH-based uptake is a type of clathrin-mediated endocytosis, together with a minor contribution of caveole-mediated endocytosis. In the same study, the authors further showed that endocytosed particles are subsequently stored in slowly acidifying vesicles of the endosomal pathway.
After our experiments of cellular uptake, we further investigated if the use of the hydrotalcite shell on the silica nanospheres has any effect on the cells viability. Previous studies showed that proximal tubular cell lines, among them the HK-2 cell line, were sensitive to the oxidative stress provoked by silica NPs.34
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium reduction assay results (Fig. 5) indicate a progressive decrease of the HK-2 cells mitochondrial activity by increasing the number of QD@silica nanoparticles (32 nm, 15 nM). Similar remarks have been reported for Human Umbilical Vein Endothelial Cells (HUVEC) cells, also an epithelial cell line, where the silica nanoparticles led to HUVEC cell death, while HeLa cells survived, indicating therefore that HUVEC are highly sensitive to silica exposure.35 In clear contrast, the hydrotalcite-coated particles (QD@silica@LDH) exhibited highly satisfactory cell viability in time, leading to the conclusion that the presence of hydrotalcite reduces the cytotoxicity of HK-2 cells.
On the other hand, the uptake kinetics showed again a cell type-dependent behavior (Fig. 6), in good agreement with previous studies on silica NPs in different cell types.35,36 Moreover, the longer the time of cell exposure to the LDH hybrids, the greater the cellular uptake. Within the first 4 h, the mean number of internalized nanoparticles increased almost linearly for both cell types. Interestingly, after 10 h, the situation was reversed and HeLa cells internalized a larger number of nanoparticles than HK-2. Alike behavior was observed for the HUVEC cells upon exposure to silica nanoparticles, thus highlighting that different cellular characteristics, as each cell type has an individual surface property and cellular morphology, display different nanoparticle–cell interactions and uptake processes.
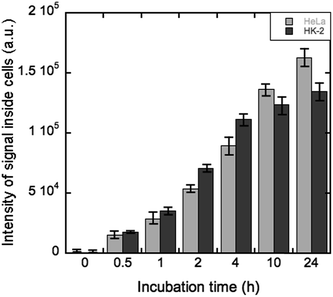 | ||
| Fig. 6 Uptake kinetics of QD@silica@LDH nanoparticles in HeLa (grey) and HK-2 cells (black). The histograms depict the mean standard error of at least three independent experiments (n = 30). | ||
Conclusions
In summary, the combination QD-silica-LDH has proved to be an effective tactic to avoid the endosomal escape and thereby to preserve the photoluminescence intensity of QDs by increasing the stability and lifetime of QD@silica. Herein, we demonstrated the potential application of highly stable and luminescent Cd-free QD@silica@layered double hydroxide nanospheres for bioimaging. The quick cellular uptake is due to the attractive interactions between the positively charged LDH nanosheets and the negatively charged cell membrane, without further functionalization. Nanoparticles of 32 nm where uniformly distributed in the cells, indicating this is the optimal size when surrounded by LDH. The coating layer confers a highly biocompatible attribute to the silica NPs, otherwise proved to be toxic by themselves when released into the HK-2, additionally acting like a barrier against degradation. Remarkably, the hydrotalcite shell is a clever strategy to enhance the endosomal escape and thereby increase the stability and lifetime of QD@silica, outstanding features for any bioimaging system.Acknowledgements
We would like to thank ICIQ, TIGEM Dulbecco Telethon Institute International Mobility for Postdoctoral Research Training (DTI-IMPORT) and Spanish MINECO through Severo Ochoa Excellence Accreditation 2014–2018 (SEV-2013-0319) for the financial support. The research leading to these results has received funding also from “la Caixa” Foundation. We are also grateful to Dr Fernando Bravo (ICIQ CSOL technology unit), and Dr Maria Antonietta De Matteis, Dr Rossella Venditti and Dr Leopoldo Staiano (TIGEM).Notes and references
- L. Hu, Z. W. Mao and C. Y. Gao, J. Mater. Chem., 2009, 19, 3108 RSC.
- D. Peer, J. M. Karp, S. Hong, O. C. Farokhzal, R. Margalit and R. Langer, Nat. Nanotechnol., 2007, 2, 751 CrossRef CAS PubMed.
- H. Lord and S. O. Kelley, J. Mater. Chem., 2009, 19, 3127 RSC.
- G. J. Nohyek, J. Lademann, C. Ribaud and M. S. Roberts, Crit. Rev. Toxicol., 2007, 37, 251 CrossRef PubMed.
- S. K. Shukla, Adv. Mater. Rev., 2014, 1, 2 Search PubMed.
- S. J. Soenen, B. B. Manshian, T. Aubert, U. Himmelreich, J. Demeester, S. C. De Smedt, Z. Hens and K. Braeckmans, Chem. Res. Toxicol., 2014, 27(6), 1050 CrossRef CAS PubMed; P. G. Luo, S. Sahu, S. T. Yang, S. K. Sonkar, J. Wang, H. Wang, G. E. LeCroy, L. Cao and Y. P. Sun, J. Mater. Chem. B, 2013, 1, 2116 RSC.
- Q. Liang, W. Ma, Y. Shi, Z. Li and X. Yang, Carbon, 2013, 60, 421 CrossRef CAS.
- M. Bacon, S. J. Bradley and T. Nann, Part. Part. Syst. Charact., 2014, 31, 415 CrossRef CAS.
- Q. Ma, I. Castelló Serrano and E. Palomares, Chem. Commun., 2011, 47, 7071 RSC.
- L. Li, W. Gu, J. Liu, S. Yan and Z. P. Xu, Nano Res., 2015, 8, 682 CrossRef CAS.
- K. Ladewig, Z. P. Xu and G. Q. Lu, Expert Opin. Drug Delivery, 2009, 6(9), 907 CrossRef CAS PubMed.
- K. Fent, C. J. Weisbrod, A. Wirth-Heller and U. Pieles, Aquat. Toxicol., 2010, 100, 218 CrossRef CAS PubMed.
- Q. Mu, N. S. Hondow, L. Krzeminski, A. P. Brown, L. J. C. Jueken and M. N. Routledge, Part. Fibre Toxicol., 2012, 9, 29 CrossRef CAS PubMed.
- I. L. Hsiao, A. M. Gramatke, R. Joksimovic, M. Sokolowski, M. Gradzielski and A. Haase, J. Nanomed. Nanotechnol., 2014, 5, 6 Search PubMed.
- Z. P. Xu, Q. H. Zeng, G. Q. Lu and A. B. Yu, Chem. Eng. Sci., 2005, 61(3), 1027 CrossRef.
- J. H. Choy, S. Y. Hwak, Y. J. Jeong and J. S. Park, Angew. Chem., Int. Ed., 2000, 39(22), 4041 CrossRef.
- J. M. Oh, S. J. Choi, G. E. Lee, J. E. Kim and J. H. Choy, Chem.–Asian J., 2009, 4, 67 CrossRef CAS PubMed.
- J. M. Oh, S. J. Choi, S. T. Kim and J. H. Choy, Bioconjugate Chem., 2006, 17, 1411 CrossRef CAS PubMed.
- G. Stoica, I. Castelló Serrano, A. Figuerola, I. Ugarte, R. Pacios and E. Palomares, Nanoscale, 2012, 4, 5409 RSC.
- B. Koean, Chem. Rev., 2009, 109, 4283 CrossRef PubMed.
- I. Castelló Serrano, G. Stoica, A. Figuerola and E. Palomares, J. Mater. Chem. B, 2013, 1, 793 RSC.
- S. Cho, S. Jung, S. Jeong, J. Bang, J. Park, Y. Park and S. Kim, Langmuir, 2013, 29(1), 441 CrossRef CAS PubMed.
- M. P. Desai, V. Labhasetwar, E. Walter, R. J. Levy and G. L. Amidon, Pharmaceut. Res., 1997, 14, 1568 CrossRef CAS PubMed.
- S. Prahba, W. Z. Zhou, J. Panyam and V. Labhasetwar, Int. J. Pharmacol., 2002, 244, 105 CrossRef.
- C. Chen, P. Wang, T. T. Lim, L. Liu, S. Liu and R. Xu, J. Mater. Chem. A, 2013, 1, 3877 CAS.
- I. Castelló Serrano, Q. Ma and E. Palomares, J. Mater. Chem., 2011, 21, 17673 RSC.
- G. Beaune, S. Tamang, A. Bernardin, P. Bayle-Guillemaud, D. Phenel, G. Schoehn, F. Vinet, P. Reiss and I. Texler, ChemPhysChem, 2011, 12, 2189 CrossRef.
- S. Massadeh and T. Nann, Nanomater. Nanotechnol., 2014, 4, 15 Search PubMed.
- N. Iyi, Y. Ebina and T. Sasaki, J. Mater. Chem., 2011, 21, 8085 RSC.
- Y. Wang, Y. Zhou, T. Zhang, M. He and X. Bu, RSC Adv., 2014, 4, 29968 RSC.
- F. Lu, S. H. Wu, Y. Hung and C. Y. Mou, Small, 2009, 5(12), 1408 CrossRef CAS PubMed.
- L. Treuel, X. Jiang and G. U. Nienhaus, J. R. Soc., Interface, 2013, 10(82), 20120939 CrossRef PubMed.
- Z. P. Xu, Q. H. Zeng, G. Q. Lu and A. B. Yu, Chem. Eng. Sci., 2005, 61(3), 1027 CrossRef.
- I. Passagne, M. Morille, M. Rousset, I. Pujalte and B. L'azou, Toxicology, 2012, 299, 112 CrossRef CAS PubMed.
- J. Blechinger, A. T. Bauer, A. A. Torrano, C. Gorzelanny, C. Bräuchle and S. W. Schneider, Small, 2013, 9(23), 3970 CrossRef CAS PubMed.
- J. Patel and A. Patel, Toxicity of Nanomaterials on the Liver, Kidney, and Spleen, Biointeractions of Nanomaterials, ed. B. Sutariya and Y. Pathak, CRC Press, 2014, pp. 285–314 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods: synthesis of NPs and treatments; NPs preparation, cell incubation; inmunostaining; MTT test; instrumentation. And results: TEM images of different-sized QDs@silica nanospheres: (a) 17 nm, (b) 27 nm, (c) 32 nm, (d) 40 nm, (e) 55 nm and (f) 65 nm; absorption and emission spectra of InP/ZnS quantum dots. See DOI: 10.1039/c6ra02497a |
| This journal is © The Royal Society of Chemistry 2016 |