Bioactive calcium phosphate cement with excellent injectability, mineralization capacity and drug-delivery properties for dental biomimetic reconstruction and minimum intervention therapy
Yue Sa†
ab,
Yixue Gao†b,
Man Wangb,
Tianfeng Wangb,
Xiaowei Fengb,
Zhejun Wangab,
Yining Wangab and
Tao Jiang*ab
aDepartment of Prosthodontics, Hospital of Stomatology, Wuhan University, 237 Luoyu Road, Wuhan 430079, PR China. E-mail: jiangtao2006@whu.edu.cn; Fax: +86 27 87873260; Tel: +86 27 87686318
bThe State Key Laboratory Breeding Base of Basic Science of Stomatology (Hubei-MOST) & Key Laboratory of Oral Biomedicine Ministry of Education, School & Hospital of Stomatology, Wuhan University, 237 Luoyu Road, Wuhan 430079, PR China
First published on 26th February 2016
Abstract
When the enamel layer is breached due to external physical and chemical reasons, the underlying dentin is exposed to a wet and bacteria-laden oral environment. Accordingly, some diseases related to exposed dentin, such as dentin hypersensitivity and bacterial invasion, usually occur and affect patients' day-to-day lives. The aim of this study was to evaluate the effectiveness of injectable calcium phosphate cement (CPC) on occluding dentinal tubules and antibacterial properties when loaded with chlorhexidine (CHX) under a simulated oral environment, which was believed to be beneficial for dental biomimetic reconstruction and minimum intervention therapy. The particle size, surface morphology and composition of CPC were characterized using scanning electron microscopy (SEM) and X-ray diffraction (XRD). The apatite formation ability, occluding effects, drug delivery and antibacterial properties of CPC and CHX-loaded CPC were further investigated using non-destructive attenuated total reflection infrared (ATR-IR) spectroscopy, Raman spectroscopy, SEM observation, permeability test, UV analysis and a disk-diffusion method. The results showed that both CPC and CHX-loaded CPC could continually form enamel-like apatite layers on the exposed dentin surface. After facing an acidic environment, the apatite layer still effectively occluded the dentinal tubules. Furthermore, CHX loaded CPC showed a sustained release of CHX over a timeframe of a week and revealed significant antibacterial effect compared to the blank control without CHX. Therefore, the results suggest that due to the unique self-setting ability, injectability, apatite-mineralization capacity and similar composition to a tooth, CPC could be used as a promising biomaterial to reconstruct the breached enamel on exposed dentin through a biomimetic and minimally invasive way. Moreover, due to the excellent drug-delivery property, CPC could easily carry antibiotics to inhibit the bacteria causing further pulp infection.
1. Introduction
Enamel is the exterior layer of the mammalian tooth consisting of approximately 97% hydroxyapatite (HA) crystals, 1% organic material and 2% water.1,2 Dentin is a tubular structure beneath the enamel layer. Both enamel and dentin are exquisite examples of biomineralized tissues. They together constitute the major component of teeth protecting the underlying pulp tissue.3–5 Unlike other biomaterials, however, enamel is a non-living tissue and has no capacity to regenerate, self-repair or remodel.6,7 Consequently, when the overlying enamel layer is breached due to external physical and chemical reasons, such as abrasion, attrition, erosion or caries, dentin is exposed to a wet and bacteria-laden oral environment where the exposed dentinal tubules open channels for the permeation of solutes, irritants and bacteria.5 As a result, several diseases related to the exposed dentin tubules occur and affect patients' day-to-day lives.Dentin hypersensitivity, defined as a short and sharp pain, is a rising dental problem in recent years caused by the loss of the enamel layer and exposure of dentinal tubules.5,8 The mostly accepted hydrodynamic hypothesis proposed by Brannstrom et al. proposes that chemical, tactile or thermal stimuli provoke the movement of dentin fluid within the exposed tubules and result in the painful sensation.9 Therefore, occluding exposed dentinal tubules is suggested to be a crucial strategy for reducing stimuli-evoked fluid shifts to desensitize dentin. Apart from dentin hypersensitivity, bacterial invasion of dentinal tubules is another commonly occurring phenomenon when dentin is exposed.10 If no effective interference is applied in time, bacterial products will continually diffuse through the dentinal tubule toward the pulp and evoke inflammatory changes in the pulpo–dentin complex.10 It is well known that cutting off the invasion pathway of bacteria and then locally delivering the antibacterial drug could effectively control the bacterial infection at an early stage. Consequently, occluding exposed dentinal tubules with a drug-delivery material is suggested as an effective way to prevent pulp infection.
Over the decades, numerous attempts have been made to occlude dentinal tubules. For instance, potassium oxalate, sodium fluoride or even some desensitizing toothpastes (Novamin, Sensodyne Freshmint and Colgate Sensitive) were developed to relieve dentin hypersensitivity.8,9,11–14 But until now, none of them are accepted as a consistent and reliable desensitizing regimen. Recently, using Ca, P materials and biomimetic approaches to repair the breached biomineralized tissues are of special interest in oral biology and medicine.5,6,15–17 It is believed that the biomimetic products are closely approximate to the compositional, structural and mechanical characteristics of biomineralized tissues. Moreover, if the formed “biomimetic layer” has antimicrobial properties, further bacterial infection will be prevented and the new caries at the margin of the restoration will be accordingly avoided.2
Calcium phosphate cements (CPCs) are a group of materials developed by Brown and Chow in the 1980s.18 Due to their excellent bioactivity, various CPC compositions have been investigated and are commercially available.19 Normally, CPCs are synthesized by mixing a solid part and a liquid part. When the mixture reaches a paste phase, it is either modeled into an open bone defect by means of a spatula or it is injected into the small and irregular bone defect by means of a syringe during a minimally invasive surgical procedure.20 The paste will then harden in situ like a “cement” and eventually transform into a hydroxyapatite.21,22 In some cases, CPCs can also be used as a carrier to load various drugs for effective infection control.20,23 Since bone and tooth have a similar composition, i.e. hydroxyapatite, it was inspired that CPCs might be used as promising biomaterials to form an “enamel-like cement” layer on the dentin surface. Such a biomimetic product is hypothesized to effectively occlude the exposed dentin tubules for desensitizing dentin. Furthermore, drug-loaded CPCs are believed to show high antimicrobial potency against early invaded bacteria in dentin tubules. However, to the best of our knowledge, no previous study has examined CPCs in such aforementioned applications.
Therefore, the purpose of this study was to evaluate the effects of a bioactive CPC system on occluding dentin tubules and delivering a drug under circumstances similar to the oral environment (artificial saliva immersion and the subsequent facing of an acidic environment). Enterococcus faecalis (E. faecalis), a bacteria that usually hides in dentin tubules,24 was selected as the bacterial model and chlorhexidine (CHX), a commonly used antibacterial agent for dental care, was selected as the drug model in the present study. To fully assess the performance of the CPC system on the exposed dentin surface, non-destructive attenuated total reflection infrared (ATR-IR) and Raman spectroscopy techniques were used to quantitatively characterize the forming capacity of the enamel-like cement layer in real-time. Scanning electron microscopy (SEM) was complementarily used to qualitatively analyze the morphological changes of the formed biomimetic layer. Furthermore, dentin permeability measurement, antibiotic release and anti-bacteria tests were used to evaluate the efficacy of dentinal tubule occlusion and infection control of the formed biomimetic layer.
2. Materials and methods
2.1 Material preparation
All experiments were performed in compliance with the relevant laws and institutional guidelines. The protocol of the experiment was approved by the local Ethics Committee of the School and Hospital of Stomatology Wuhan University, China. Forty caries-free extracted human third molars were obtained from patients after they signed informed consent. All the obtained molars were examined under 20× magnification to detect enamel cracks or fractures, carious and other defects. The teeth were cleaned thoroughly and one dentin disk with a thickness of 1.0 ± 0.1 mm was cut from each tooth with the direction perpendicular to the long axis of the tooth above the cemento-enamel junction by means of a low-speed water cooled diamond saw (Isomet, Buehler, Lake Bluff, IL, USA). Each disk was carefully prepared and inspected to ensure that they were free of coronal enamel or pulpal exposures. After that, a standard smear layer was created on both sides of the dentin specimens using 600-grit silicon carbide paper for 30 s under constant water irrigation.CPC particles were composed of a phase mixture of 61% α-TCP, 26% CaHPO4, 10% CaCO3 and 3% precipitated HA (Calcibon®, Biomet Merck, Darmstadt, Germany). The morphological investigation of the CPC particles was performed using a scanning electron microscope (S-4800, Hitachi). The crystal phase of the CPC was identified using X-ray diffraction (XRD, X′ Pert Pro, The Netherlands) with a wavelength of 1.5406 Å at a voltage of 40 kV and a current of 40 mA. XRD patterns were collected for 2θ values between 10° and 80° in a continuous mode at a rate of 25 seconds per step and a step size of 0.026° (2θ).
To load CHX, commercial CHX solution (Sigma Chemical Co) was firstly diluted by artificial saliva (AS) to a final weight concentration of 2%, 0.2% and 0.02%. Then, 50 mg of CPC power was soaked into 2 ml different concentrations (0.02%, 0.2%, 2%) of CHX-AS solution at 4 °C overnight to load CHX. After soaking, the powders were separated by centrifuging at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min and the supernatant was completely removed. The CHX-loaded CPC were gently flushed with distilled water (DW) to remove the unloaded CHX and then dried at 37 °C overnight to obtain the CHX-loaded CPC powder.
000 rpm for 10 min and the supernatant was completely removed. The CHX-loaded CPC were gently flushed with distilled water (DW) to remove the unloaded CHX and then dried at 37 °C overnight to obtain the CHX-loaded CPC powder.
2.2 Experimental design
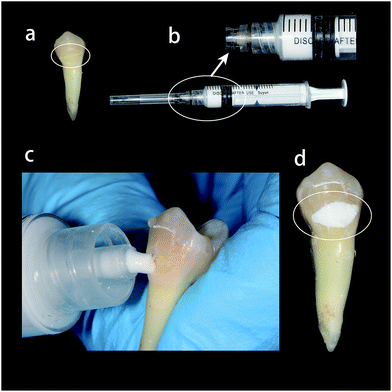
The experimental design is summarized in Fig. 1. Specifically, the procedure of CPC application on the exposed dentin surface is summarized in Fig. 1a. The antibiotic release and anti-bacterial test of CHX-loaded CPC are summarized in Fig. 1b. | ||
| Fig. 1 Summary of the experimental design for different treatments and measurements. (a) Dentin surface remineralization test; (b) antibiotic release test and anti-bacteria test. | ||
After material preparation, all of the dentin disks were immersed in 0.5 M EDTA solution (pH 7.4) for 5 min. The etched disk was rinsed and kept wet to evaluate the maximum permeability. After that, the dentin disks were randomly divided into four principal groups (n = 10) with consideration of baseline permeability:
DW group: dentin disks were stored in distilled water (DW) as a control.
AS group: dentin disks were stored in artificial saliva (AS).
CPC-AS group: dentin disks were slightly rubbed with CPC paste for 1 min and then stored in AS.
CPC-2% CHX-AS group: dentin disks were slightly rubbed with 2% CHX loaded CPC paste for 1 min and then stored in AS.
The compositions of the AS were 1.5 mmol l−1 CaCl2, 50 mmol l−1 KCl, 0.9 mmol l−1 KH2PO4 and 20 mmol l−1 Tris with pH was adjusted to 7.4. The CPC paste was obtained by mixing 1 g of CPC powder with 0.35 ml of the 4% disodium hydrogen phosphate (Na2HPO4) solution in a liquid-to-powder ratio of 0.35.25 The obtained CPC paste was injected using a syringe to the dentin disk and then slightly spread with a dental spatula for 1 min. After self-setting at room temperature for around 5 min,26 residual CPC was rinsed by DW (Fig. 2). Treated dentin disks were immersed in 50 ml AS or DW for 7 days at 37 °C and the solutions were replaced every 24 h with fresh ones.
2.3 Dentin permeability evaluation
Dentin permeability was evaluated using a fluid filtration system in a modified split-chamber unit at a simulated pulpal pressure of 20 cm H2O according to our previous studies.8,9 Briefly, ten dentin disks were used for the dentin permeability measurement in each group. The permeability of each specimen was expressed as a percentage (Lp%) of the fluid flow through the EDTA-etched dentin disk of the same specimen. Measurements were performed after EDTA etching and after 7 days treatments. The maximum permeability which was after EDTA etching was assigned to a value of 100% permeability.2.4 ATR-IR spectroscopy
ATR-IR spectra were obtained from dentin disks after EDTA etching, 1 day, 3 day and 7 day storage using a Nicolet 5700 FTIR spectrophotometer (Nicolet, Madison, WI, USA) equipped with a diamond crystal ATR accessory. To reflect the real-time changes of dentin disks after treatment, reference points were marked on the surface of each specimen. Then spectra were collected in the range of 800–1800 cm−1, with a 4 cm−1 resolution for a total of 64 scans, and analyzed by OMNIC 8 software. Each specimen was analyzed in three different positions before and after treatment. The spectrum acquired at the end of the analyses represented the average of the three single scans. A spectrum of water was obtained and subtracted from each of the original spectra which were then processed by smoothing, baseline corrected, and normalized to the amide I peak.27 The mineral matrix ratio (the ratio of integrated areas of the phosphate v1, v3 stretching mode to the amide I peak) was measured in all spectra in order to quantitatively calculate the extent of mineral changes.282.5 Raman spectroscopy
Raman spectra were acquired from dentin disks after EDTA etching, subsequent to 1 day, 3 day and 7 day storage using a micro-Raman spectrometer (i-Raman Portable Raman Spectrometer, B&W TEK Inc., USA) equipped with a semiconductor laser diode at a 785 nm wavelength. Each specimen was analyzed in three points, using a 0–3200 cm−1 range and a 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m s−1 integration time at room temperature. BWSpec 4 spectroscopic software (BWSpec, B&W TEK Inc.) was used to analyze the acquired spectra. Original spectra were baseline corrected and smoothed to avoid laser-induced fluorescence. For the quantitative analysis of Raman results during the treatment, the intensity and full width at half maximum (FWHM) of the strongest v1 PO43− peak at 960 cm−1 was recorded and calculated from the Raman spectra according to previous investigations.29–32
000 m s−1 integration time at room temperature. BWSpec 4 spectroscopic software (BWSpec, B&W TEK Inc.) was used to analyze the acquired spectra. Original spectra were baseline corrected and smoothed to avoid laser-induced fluorescence. For the quantitative analysis of Raman results during the treatment, the intensity and full width at half maximum (FWHM) of the strongest v1 PO43− peak at 960 cm−1 was recorded and calculated from the Raman spectra according to previous investigations.29–32
2.6 SEM observation
The surface morphology for dentin disks following the 7 day treatment and subsequent acid challenge were observed using SEM. Samples were dried and sputter-coated with platinum (E1010, Hitachi, Japan), then observed with a field emission SEM (S-4800, Hitachi, Japan) at 10 kV.2.7 In vitro antibiotic release test
The loading amount of CHX was determined using UV analysis (at wavelength 254 nm) through calculating the difference of CHX-AS solution before and after loading. For the CHX release test, 1 g of the collected CHX-loaded CPC powder was mixed with 0.35 ml of Na2HPO4 solution. After setting the reaction in room temperature, it was soaked into 50 ml fresh AS at 37 °C for different periods of time. At each time point, 2 ml of AS solution was taken out to test the released CHX and 2 ml of fresh AS was added back. The accumulative release percentage of CHX from CPC disk was then calculated.2.8 In vitro anti-bacterial test
A disk-diffusion method was used to investigate the inhibitory effect of the CHX-CPC against E. faecalis. 30 μl of the E. faecalis suspensions over the range of 0.05–2 mg ml−1 were pipetted onto petri dishes containing brain–heart infusion broth culture medium and then distributed evenly. Different CHX-loaded CPC powder was separately mixed with Na2HPO4 solution with the ratio of 1 g to 0.35 ml. Then the paste was injected into a Teflon mold (diameter = 13.0 mm and height = 1.0 mm). After setting the reaction in room temperature, it was placed onto the semi-solid culture medium for 24 hours incubation at 37 °C. After that, the anti-bacterial activities of the prepared CHX-loaded CPC powder was evaluated by measuring the zone of inhibition (ZOI), which was defined as the clear region around the disk saturated with an antimicrobial agent on the agar surface. Each sample was repeated three times.2.9 Statistical analysis
Statistical analyses were performed with SPSS 19.0 (SPSS, Chicago, IL, USA). Two-way repeated measures analysis of variance (ANOVA) was applied to evaluate the ATR-IR, Raman and dentin permeability results, considering the treatment as the main effect and treatment time as the repeated measure at a 5% significance level. One-way ANOVA with a post hoc Tukey test was used to analyze the ZOI values.3. Results
3.1 Material characterization
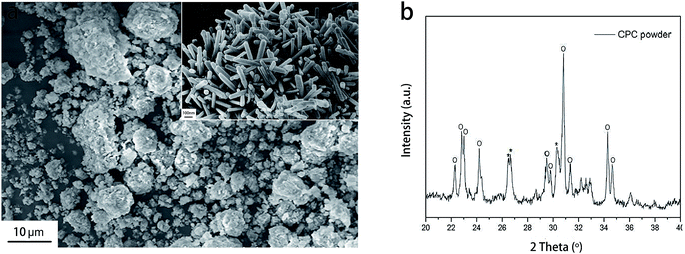
The SEM analysis shows sphere-like CPC powder with approximate particle sizes of 1–30 μm at low magnification (1500×). Furthermore, dispersive clusters of crystals are observed on the surface of the CPC powder at high magnification (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×), leaving numerous nano-sized pores among crystals (Fig. 3a). Complementing XRD analysis demonstrates that the CPC powder is mainly composed of α-TCP (Fig. 3b).
000×), leaving numerous nano-sized pores among crystals (Fig. 3a). Complementing XRD analysis demonstrates that the CPC powder is mainly composed of α-TCP (Fig. 3b).
3.2 ATR-IR spectroscopy
All representative spectra are recorded in the region of 800–1800 cm−1. Bands at 885–1180 cm−1, representative of mineral components, is assigned to the v1, v3 PO43−, and bands from 1600 to 1725 cm−1, representative of organic components, is assigned to Amide I. It is found that the v1, v3 PO43− peak remarkably increased during the 7 day period in the CPC-AS and CPC-2% CHX-AS groups. However, few changes in the v1, v3 PO43− peak could be observed in the DW and AS groups during the 7 day immersion (Fig. 4a–d). Two-way repeated ANOVA and Tukey's multiple comparison tests illustrated a statistically significant main effect for both time and treatment and their interactions on the mineral matrix area ratio (p < 0.001, p < 0.001, and p < 0.001, respectively). Specifically, the mineral matrix area ratio of the CPC-AS group and CPC-2% CHX-AS group increased significantly from 5.39 up to 14.46 and from 5.30 to 13.81 after 7 day AS immersion, respectively (p = 0.001 and p < 0.001, respectively). In contrast, the mineral matrix area ratio of the DW or AS groups remained almost unchanged after 7 day immersion (p > 0.05 and p > 0.05, respectively) (Fig. 4e). The v1, v3 PO43− peak of the CPC-AS and CPC-2% CHX-AS groups increased significantly compared to that of the DW or AS groups after 7 day immersion. No significant differences were found between the DW and AS groups after 7 day immersion. After facing an acidic environment for 1 min, there was no significant change in the mineral matrix area ratio in the DW, AS and CPC-2% CHX-AS groups (p > 0.05, p > 0.05 and p > 0.05, respectively). But a significant decrease of the mineral matrix area ratio in the CPC-AS group was observed (p = 0.039).3.3 Raman spectroscopy
The main features of the Raman scattering spectra are shown in Fig. 5. The strongest peak at 960 cm−1 is attributed to v1 PO43−. The peaks at 1045 and 1024 cm−1 are assigned to v3 PO43−. The typical peak at 1068 cm−1 arises from v3 CO32−. Two-way repeated ANOVA analysis revealed that a statistically significant main effect on the intensity of the v1 PO43− value for both time and treatment and their interactions (p < 0.001). The intensity of v1 PO43− increased significantly in the CPC-AS group and the CPC-2% CHX-AS group after 7 day AS immersion (p = 0.001 and p = 0.001, respectively), whereas it remained stable in the other two groups (p > 0.05) (Fig. 5a–d). After facing an acidic environment for 1 min, there was no significance of the intensity of v1 PO43− in any of the groups (p > 0.05).The FWHM of the v1 PO43− peak is shown in Table 1. After 7 day immersion, the FWHM of the v1 PO43− peak stayed relatively stable in the DW and AS groups (p > 0.05 and p > 0.05, respectively) but increased significantly in the CPC-AS group and CPC-2% CHX-AS group (p = 0.026 and p = 0.039, respectively). After facing an acidic environment, FWHM of the v1 PO43− peak decreased in all groups but no significant difference was found in any of the groups (p > 0.05).
| Groups | DW | AS | CPC-AS | CPC-2% CHX-AS |
|---|---|---|---|---|
| Baseline | 15.326(0.14) | 15.431(0.40) | 15.164(0.12) | 15.311(0.12) |
| 1 day mineralization | 15.348(0.26) | 15.432(0.47) | 15.010(0.15) | 16.004(0.10) |
| 3 day mineralization | 15.378(0.25) | 15.398(0.34) | 16.329(0.07) | 16.265(0.13) |
| 7 day mineralization | 15.350(0.20) | 15.506(0.35) | 16.931(0.21) | 16.685(0.20) |
| After facing an acidic environment | 15.344(0.23) | 15.431(0.32) | 16.799(0.33) | 16.620(0.19) |
3.4 SEM analysis
After EDTA treatment, dentin surfaces are free of a smear layer and nearly all of the dentinal tubules are open in the DW group. Only a few mineral-like deposits are found on the dentin surface in the AS group. But almost all dentinal tubules are occluded by a layer of crystal-like deposits in the CPC-AS group and the CPC-2% CHX-AS group (Fig. 6). After facing an acidic environment, completely exposed dentin surfaces with open tubules are displayed in both the DW group and the AS group. In contrast, relatively flat but apatite layers are left on the surface of dentin disk in the CPC-AS group and the CPC-2% CHX-AS group, which still effectively occlude the dentinal tubules (Fig. 7).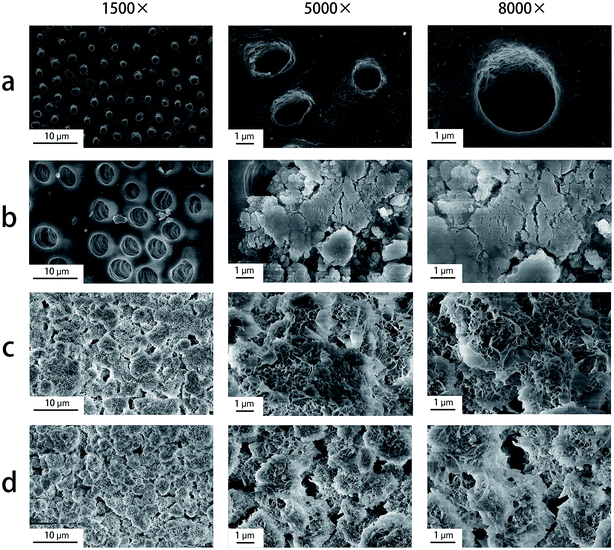 | ||
| Fig. 6 Dentin surface morphology by different treatments after 7 days immersion. (a) DW group; (b) AS group; (c) CPC-AS group; (d) CPC-2% CHX-AS group. | ||
 | ||
| Fig. 7 Dentin surface morphology of different groups after facing an acidic environment. (a) DW group; (b) AS group; (c) CPC-AS group; (d) CPC-2% CHX-AS group. | ||
3.5 Dentin permeability measurements
The dentin permeability results are expressed as percentages of the maximum permeability considered to be equal to 100% by EDTA etching. Table 2 shows the data of dentin permeability produced by four different treatments. Statistical analyses show that CPC-AS and CPC-2% CHX-AS treatments significantly reduced the dentin permeability (p < 0.001 and p < 0.001, respectively), but DW and AS immersion did not induce a significant difference on dentin permeability (p > 0.05 and p > 0.05, respectively). After facing an acidic environment, the dentin permeability of all four groups had increased. Statistical significances were found in the AS, CPC-AS and CPC-2% CHX-AS groups (p = 0.006, p = 0.038 and p = 0.009, respectively). No significance was found in the DW group by citric acid treatment (p > 0.05).| Treatments | No treatment | DW | AS | CPC-AS | CPC-2% CHX-AS |
|---|---|---|---|---|---|
| a The values (expressed as %) are reported as mean ± standard deviation. Lp after EDTA treatment represented the maximum permeability (Lp = 100%). | |||||
| EDTA application | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 7-day mineralization | 101.3 ± 2.7 | 99.8 ± 5.7 | 92.2 ± 6.2 | 29.9 ± 8.3 | 32.1 ± 7.9 |
| Acid challenge | 102.7 ± 8.1 | 101.5 ± 10.2 | 97.1 ± 7.2 | 41.9 ± 11.3 | 52.6 ± 9.2 |
3.6 In vitro antibiotic release
CHX in CPC powder was found to reveal a sustained release profile in AS (Fig. 8). There was no significant difference in the loading efficiencies of CPC powder loaded with three concentrations of CHX (0.02%, 0.2% and 2%, respectively) (p > 0.05). All of them revealed high CHX loading efficiencies of around 70% even after one week, indicating the good drug-delivery property of CPC. | ||
| Fig. 8 Accumulative release of CHX from the CPC with different concentrations over a week (the data represented is mean and standard deviation). | ||
3.7 In vitro anti-bacterial ability
The inhibition abilities of CHX-loaded CPC are shown in Fig. 9. It was found that all the measured plates showed a translucent zone against E. faecalis. Greater diameter of ZOI is found in the increased concentration of CHX in CPC. Statistical significance was found between all of the groups (p < 0.001). | ||
| Fig. 9 Antibacterial activity of disk-like samples by zone of inhibition (ZOI) test against E. faecalis. Translucent zones indicated inhibition of bacterial growth. | ||
4. Discussion
CPC, a highly bioactive CaP-based bone substitute, has been widely used for trauma and orthopaedic surgery for many years.33 During surgery, surgeons inject CPC to meet the specific demand of irregular bone defects. Normally CPC can be classified into apatite-forming and brushite-forming CPC, based upon the setting product of the CPC.34 Calcibon® is the commercially available apatite-forming CPC.26 Although Calcibon® was originally developed as a bone conductive material, it may also form an apatite layer on the surface of dentin which shares a similar structure and composition with bone. Furthermore, the injectability of CPC could avoid the unnecessary cut of dental structure to reconstruct the breached enamel with minimum intervention therapy.ATR-IR and Raman spectroscopies were applied to quantitatively monitor the presence of apatite formation of CPC on the surface of exposed dentin. The advantage of these two spectroscopic methods is the non-destructive approach which provides evaluation of the apatite formation in real time.27,35,36 Unlike conventional FTIR, which needs sample to be scraped off and ground into fine powder, ATR-IR and Raman spectroscopy permit repeated analyses on the same place of the intact specimen surface, thus ensuring high comparability between spectra before and after treatment. ATR-IR spectroscopy has the advantage that examining biological specimens entails no problems with fluorescence and Raman spectroscopy exhibits less interference from water than ATR-IR. Furthermore, the penetration depth of the IR beam is 0.5–5 μm, while the laser diode used for the Raman analysis may have a deeper penetration (>5 μm) in dental tissues.37 Thus, when both techniques are combined, a higher level of accuracy in the apatite-forming evaluation was offered as ATR-IR analysis provided information on the apatite content changes in superficial dentin and Raman analysis, on the other hand, complementarily provided the same information but from deeper dentin.
In the present study, we found the mineral matrix area ratio from ATR-IR spectra and the intensity of v1 PO43− from Raman spectra increased dramatically during the 7 days immersing period in two CPC containing groups, which directly confirmed the continued formation of apatite crystals on the dentin surface. Furthermore, the mineral matrix area ratio and intensity of v1 PO43− in two of the CPC containing groups began to decrease after facing an acidic environment. However, due to the relatively short treatment time and the different detection limits of ATR-IR and Raman, only a decreased mineral matrix area ratio in the CPC-AS group was observed by ATR-IR. This result suggests facing an acidic environment weakened the formed apatite crystals in the CPC-AS group.
Besides mineral matrix area ratio and intensity of v1 PO43−, it should be noted that the FWHM of the v1 PO43− peak from the Raman spectra and the contour for v1, v3 PO43− from the ATR-IR spectra became wider in both CPC containing groups during the 7 day immersing period, which indicates the decrease of crystallinity of the formed apatite in these groups. After facing an acidic environment, the FWHM of the v1 PO43− peak and contour for v1, v3 PO43− became sharper again in both CPC containing groups. It seemed that the crystallinity of the formed apatite started to increase in these groups. But due to the short time of exposure to an acidic environment, no significant increase of crystallinity was found. These interesting phenomena might be explained by the existence of a structured hydrated layer in freshly formed apatite crystals.38–40 Such a hydrated layer corresponds to the “non-apatitic environments”, which is one of the most important characteristics of biologically poorly crystalline apatites in dentin and bone. These environments are believed to be mainly located at the surfaces of the apatite crystals, while the core of the apatite crystals may contain the relatively ordered and stable apatite domains. Thus, newly formed apatite showed decreased crystallinity due to the surrounding non-apatitic hydrated layer. When faced with an acidic environment, the hydrated layer began to fade away. The ordered and stable core would be mainly responsible for the crystallinity of the apatite, which started to increase the outcome.
In support of the quantitative molecular evidence from ATR-IR and Raman spectra, SEM examinations clearly showed the morphological changes on the dentin surface in a qualitative way, from which open dentinal tubules in two CPC-free groups (a few mineral deposits from solution can be found in the AS group) but an enamel-like layer of apatite on the dentin surface in the other two CPC-containing groups after 7 days treatment can be observed. These results clearly prove the excellent occluding effects of the formed apatite from CPC. Further comparing the CPC-AS group and CPC-2% CHX-AS group, it is noted that there is no morphological difference of the apatite layer between these two groups, indicating the loaded CHX did not interfere with the transformation of CPC products into apatite. Dentin permeability results supported the SEM findings. After 7 days treatment, the DW group and AS group showed Lp% values of 99.8 and 95.2, respectively. In contrast, due to the occluding effect of formed apatite, the CPC-AS group and the CPC-2% CHX-AS group revealed significantly decreased Lp% values of 29.9 and 32.1, respectively.
Citric acid, which is regarded as a common component of fruit and soft drinks, has been widely used previously to explore the erosion mechanisms of enamel and dentin.8,41–43 Therefore, citric acid was chosen in this study as a post-treatment to simulate oral environment. It is interesting to note that apatite products in both the CPC-AS group and CPC-2% CHX-AS group still blocked most of the dentinal tubules after exposure to an acidic environment. In contrast, it is evident that most of the precipitates were solubilized in the DW group and the AS group after acid treatment, leaving the dentin surface and dentinal tubules completely exposed. Dentin permeability results showed agreement with the SEM observation. After acid treatment, Lp% values in the CPC-AS group and the CPC-2% CHX-AS group still stayed as 41.9 and 52.6, respectively. However, Lp% values in the DW group and AS group increased to 102.7 and 101.5, respectively. These results further demonstrate that the setting products of CPC show significant resistance to acid attack in a simulated oral environment.
Besides the apatite-mineralization ability, CPC can be efficiently loaded with differently concentrated antibiotic CHX and these CHX in CPC all revealed sustained releases in AS. Even after two weeks, the accumulative release of CHX was around 70%, indicating the excellent drug-delivery property of CPC. Furthermore, it is interesting to find that different concentrations of CHX did not affect the release behavior of CPC. As we mentioned before, clusters of crystals endow CPC with a high surface area and numerous nano-pores among crystals. Such delicate structures in CPC are believed to show a beneficial effect on the sustained release of CHX.
The in vitro anti-bacterial effect of CPC was also evaluated in this study. Due to the inherent advantage of a low setting temperature,23 CPC did not affect the drug activity of CHX. All three concentrations of CHX showed a clear ZOI and the diameter of the ZOI became greater with the increase in concentration. Such results indicate that CPC could deliver antibiotics to inhibit bacteria growth. The loaded CHX remarkably enhanced the antibacterial effect of CPC and 2% CHX showed the greatest antibacterial effect.
In this study, we proved that an enamel-like apatite layer could be simply and successfully formed from CPC paste, the hardened apatite product could effectively occlude the exposed dentinal tubules and deliver a drug to inhibit bacterial. According to a previous study, the setting-product of Calcibon® possesses the compression strength of 34 ± 7 MPa,26 which is similar with trabecular bone showing compression strength in the range of 8–38 MPa. Therefore, the formed enamel-like layer could resist the pressure from routine mastication to some extent. Furthermore, due to the main advantages of injectability and apatite-mineralization ability, CPC could repair the irregular enamel defect through a minimally invasive way to avoid the unnecessary enamel cutting. Consequently, CPC holds the potential to be used as a promising candidate for dental biomimetic reconstruction in the dental clinic.
5. Conclusion
Due to the unique self-setting ability, injectability, apatite-mineralization capacity and similar composition with teeth, CPC could be used as a promising biomaterial to reconstruct the breached enamel on exposed dentin through a biomimetic and minimally invasive way. Moreover, due to the excellent drug-delivery property, CPC could easily carry antibiotics to inhibit the bacteria for further pulp infection.Conflict of interest
The authors deny any conflict of interest related to this study.Acknowledgements
This study was supported by the Natural Science Foundation of China (No. 81500887, No. 81470771), the Natural Science Fund of Hubei Province (No. 2013CFA068) and the Fundamental Research Funds for the Central Universities (No. 2042015kf0093, No. 2042014kf00232).Notes and references
- Y. Z. Zhou, Y. Cao, W. Liu, C. H. Chu and Q. L. Li, ACS Appl. Mater. Interfaces, 2012, 4, 6901–6910 CAS.
- Q. Ruan, Y. Zhang, X. Yang, S. Nutt and J. Moradian-Oldak, Acta Biomater., 2013, 9, 7289–7297 CrossRef CAS PubMed.
- L. Li, C. Mao, J. Wang, X. Xu, H. Pan, Y. Deng, X. Gu and R. Tang, Adv. Mater., 2011, 23, 4695–4701 CrossRef CAS PubMed.
- P. A. Fang, J. F. Conway, H. C. Margolis, J. P. Simmer and E. Beniash, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 14097–14102 CrossRef CAS PubMed.
- Y. C. Chiang, H. P. Lin, H. H. Chang, Y. W. Cheng, H. Y. Tang, W. C. Yen, P. Y. Lin, K. W. Chang and C. P. Lin, ACS Nano, 2014, 8, 12502–12513 CrossRef CAS PubMed.
- U. G. Wegst, H. Bai, E. Saiz, A. P. Tomsia and R. O. Ritchie, Nat. Mater., 2015, 14, 23–36 CrossRef CAS PubMed.
- U. G. Wegst, H. Bai, E. Saiz, A. P. Tomsia and R. O. Ritchie, Nat. Mater., 2015, 14, 23–36 CrossRef CAS PubMed.
- Z. Wang, Y. Sa, S. Sauro, H. Chen, W. Xing, X. Ma, T. Jiang and Y. Wang, J. Dent., 2010, 38, 400–410 CrossRef PubMed.
- Z. Wang, T. Jiang, S. Sauro, Y. Wang, W. Xing, S. Liang, Y. Sa, C. Zhang, Y. Shen and M. Haapasalo, Am. J. Dent., 2012, 25, 26–30 Search PubMed.
- R. M. Love and H. F. Jenkinson, Crit. Rev. Oral Biol. Med., 2002, 13, 171–183 CAS.
- J. D. Greenhill and D. H. Pashley, J. Dent. Res., 1981, 60, 686–698 CrossRef CAS PubMed.
- T. Suge, K. Ishikawa, A. Kawasaki, M. Yoshiyama, K. Asaoka and S. Ebisu, J. Dent. Res., 1995, 74, 1709–1714 CrossRef CAS PubMed.
- D. C. Clark, J. A. Hanley, S. Geoghegan and D. Vinet, J. Periodontal Res., 1985, 20, 212–219 CrossRef CAS PubMed.
- D. C. Clark, J. A. Hanley, S. Geoghegan and D. Vinet, J. Periodontal Res., 1985, 20, 212–219 CrossRef CAS PubMed.
- C. Xu, P. Su, X. Chen, Y. Meng, W. Yu, A. P. Xiang and Y. Wang, Biomaterials, 2011, 32, 1051–1058 CrossRef CAS PubMed.
- C. Xu, P. Su, X. Chen, Y. Meng, W. Yu, A. P. Xiang and Y. Wang, Biomaterials, 2011, 32, 1051–1058 CrossRef CAS PubMed.
- L. Li, H. Pan, J. Tao, X. Xu, C. Mao, X. Gu and R. Tang, J. Mater. Chem., 2008, 18, 4079 RSC.
- W. Brown and L. Chow, J. Dent. Res., 1983, 22314, 672 Search PubMed.
- J. Zhang, W. Liu, V. Schnitzler, F. Tancret and J. M. Bouler, Acta Biomater., 2014, 10, 1035–1049 CrossRef CAS PubMed.
- E. Vorndran, M. Geffers, A. Ewald, M. Lemm, B. Nies and U. Gbureck, Acta Biomater., 2013, 9, 9558–9567 CrossRef CAS PubMed.
- M. A. Lopez-Heredia, G. J. Kamphuis, P. C. Thune, F. C. Oner, J. A. Jansen and X. F. Walboomers, Biomaterials, 2011, 32, 5411–5416 CrossRef CAS PubMed.
- M. A. Lopez-Heredia, G. J. Kamphuis, P. C. Thune, F. C. Oner, J. A. Jansen and X. F. Walboomers, Biomaterials, 2011, 32, 5411–5416 CrossRef CAS PubMed.
- M.-P. Ginebra, C. Canal, M. Espanol, D. Pastorino and E. B. Montufar, Adv. Drug Delivery Rev., 2012, 64, 1090–1110 CrossRef CAS PubMed.
- M.-P. Ginebra, C. Canal, M. Espanol, D. Pastorino and E. B. Montufar, Adv. Drug Delivery Rev., 2012, 64, 1090–1110 CrossRef CAS PubMed.
- E. M. Oomsa, J. G. C. Wolkea, M. T. Heuvelb, B. Jeschkec and J. A. Jansen, Biomaterials, 2003, 24, 989–1000 CrossRef.
- J. Van der Stok, H. Weinans, N. Kops, M. Siebelt, P. Patka and E. M. Van Lieshout, Injury, 2013, 44, 1368–1374 CrossRef PubMed.
- T. Jiang, X. Ma, Y. Wang, Z. Zhu, H. Tong and J. Hu, J. Dent. Res., 2007, 86, 1040–1045 CrossRef CAS PubMed.
- Z. Wang, T. Jiang, S. Sauro, Y. Wang, I. Thompson, T. F. Watson, Y. Sa, W. Xing, Y. Shen and M. Haapasalo, J. Dent., 2011, 39, 746–756 CrossRef CAS PubMed.
- L. Sun, S. Liang, Y. Sa, Z. Wang, X. Ma, T. Jiang and Y. Wang, J. Dent., 2011, 39, 686–692 Search PubMed.
- Y. Sa, F. Yang, S. C. Leeuwenburgh, J. G. Wolke, G. Ye, J. R. de Wijn, J. A. Jansen and Y. Wang, J. Biomed. Mater. Res., Part B, 2015, 103, 548–555 CrossRef PubMed.
- Y. Sa, Z. Wang, X. Ma, C. Lei, S. Liang, L. Sun, T. Jiang and Y. Wang, J. Biomed. Opt., 2012, 17, 035002 CrossRef PubMed.
- J. J. Freeman, B. Wopenka, M. J. Silva and J. D. Pasteris, Calcif. Tissue Int., 2001, 68, 156–162 CrossRef CAS PubMed.
- S. Larsson and G. Hannink, Injury, 2011, 422, S30–S34 CrossRef PubMed.
- M. Bohner, Eur. Spine J., 2001, 102, S114–S121 Search PubMed.
- A. Carden and M. D. Morris, J. Biomed. Opt., 2000, 5, 259–268 CrossRef CAS PubMed.
- S. G. Kazarian and K. L. Chan, Biochim. Biophys. Acta, 2006, 1758, 858–867 CrossRef CAS PubMed.
- F. M. Pascon, K. R. Kantovitz, L. E. S. Soares, A. M. do Espírito Santo, A. A. Martin and R. M. Puppin-Rontani, J. Biomed. Opt., 2012, 17, 0750081–0750086 CrossRef PubMed.
- S. Cazalbou, C. Combes, D. Eichert, C. Rey and M. J. Glimcher, J. Bone Miner. Metab., 2004, 22, 310–317 CrossRef PubMed.
- D. Farlay, G. Panczer, C. Rey, P. D. Delmas and G. Boivin, J. Bone Miner. Metab., 2010, 28, 433–445 CrossRef PubMed.
- C. Rey, C. Combes, C. Drouet, H. Sfihi and A. Barroug, Mater. Sci. Eng., C, 2007, 27, 198–205 CrossRef CAS.
- A. Wiegand, A. Stock, R. Attin, C. Werner and T. Attin, J. Dent., 2007, 35, 21–27 CrossRef CAS PubMed.
- M. E. Barbour, D. M. Parker, G. C. Allen and K. D. Jandt, Eur. J. Oral Sci., 2003, 111, 258–262 CrossRef CAS PubMed.
- N. X. West, J. A. Hughes and M. Addy, J. Oral Rehabil., 2000, 27, 875–880 CrossRef CAS PubMed.
Footnote |
| † These two authors contribute equally to this paper. |
| This journal is © The Royal Society of Chemistry 2016 |