Alkynylation/dearomatizative cyclization to construct spiro[5.5]undecanes†
Jidong Shaoa,
Liqi Li*a,
Jie Zhangb,
Jingping Hua,
Jijun Xuea and
Ying Li*a
aState Key Laboratory of Applied Organic Chemistry, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000, P. R. China. E-mail: liying@lzu.edu.cn; lilq@lzu.edu.cn
bState Key Laboratory of Fluorine & Nitrogen Chemicals, Xi'an Modern Chemistry Research Institute, Xi'an, 710065, P. R. China
First published on 22nd March 2016
Abstract
A new access to spiro[5.5]undecane frameworks was reported through the ZnMe2-promoted alkynylation of salicylaldehyde and HCO2H-mediated dearomatizative cyclization which can be used to construct an all-carbon quaternary spirocenter. This method can be applied to the rapid synthesis towards a series of useful motifs of natural products, such as the core of elatol and aphidicolin.
Spirocycles1 are a common structural pattern found at the core of numerous natural products with broad structural diversities and important bioactivities (Fig. 1).2 For example, elatol displays activity against bacteria (including human pathogenic bacteria)3a,b and demonstrates cytotoxicity against HeLa and Hep-2 human carcinoma cell lines.3c Aphidicolin also exhibits significant antiviral and antitumor activity.4a,b
One commonly exploited mechanistic approach to construct spirocycle skeleton is the dearomatizative spirocyclization of functionalized phenols and naphthols. For example, chiral hypervalent iodine catalyst using m-CPBA as terminal oxidant was developed for intramolecular spirolactonization.5,6 Palladium- or iridium-catalyzed intramolecular allylic dearomatization of phenols was also described.7 Feringa et al. developed one-pot spirocyclization involving asymmetric conjugate addition and subsequent oxidative dearomatization of 2-naphthols.8 Katsuki et al. reported iron-catalyzed asymmetric tandem spirocyclization from 1-methyl-2-naphthols and phenols.9 Luan et al. and You et al. described Ru or Rh-catalyzed vinylative dearomatization of naphthols or phenols via a C(sp2)–H bond activation approach.10 Gulías et al. and Lam et al. independently reported Rh-catalyzed spiroannulation of ortho-vinylphenols triggered by terminal C–H functionalization of the alkenyl moiety.11 Luan et al. developed a Pd-catalyzed [2 + 2 + 1] annulative dearomatization between β-naphthols and two alkyne units,12 and Pd-catalyzed alkyne insertion/β-naphthol dearomatization cascade to construct spirocycles.13 Other methods were also described by various groups.14–18 However, the reported approaches are either transition-metal catalysis or their substrates are mostly limited to structurally similar cyclic lactones or lactams. Thus, new strategies for intermolecular spirocyclization using different classes of starting materials in one-pot process are highly desirable but challenging.
In 2015, we reported an approach to synthesize 2,4-difunctionalized benzopyrans in one-pot process (Scheme 1, top).19 As a continuation of our focus on this research program, the present paper describes a new access to spiro[5.5]undecane frameworks through the ZnMe2-promoted alkynylation of salicylaldehyde and HCO2H-mediated dearomatizative cyclization that can be applied to construct an all-carbon quaternary spirocenter (Scheme 1, bottom).
The present investigation began by reacting different diene substrate (3a) with 5-methoxysalicylaldehyde (1a) and 3-methoxyprop-1-yne (2a) under standard reaction conditions, which were developed in our previous research.19 We unexpectedly obtained a spirocycle product (4a) in 7% yield instead of benzopyran compound (Table 1, entry 2). The structure of 4a was identified by X-ray crystallography (Fig. 2).
| Entryb | Acid | Solvent | Temp (°C) | Yieldc (%) |
|---|---|---|---|---|
a Unless otherwise specified, all reactions were performed using Cu(OTf)2 (8 mol%), ZnMe2 (5 equiv.), 1a (1 equiv.), 2a (4 equiv.), 3a (5 equiv.), and acid (1.5 equiv.).b For all entries, dr is >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was determined by 1H NMR spectroscopy. In our experimental results, if diastereoisomers could not be detected in 1H NMR spectra, we identified the dr as >20 1, which was determined by 1H NMR spectroscopy. In our experimental results, if diastereoisomers could not be detected in 1H NMR spectra, we identified the dr as >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.c Yield of isolated product.d (R)-(−)-1,1′-Binaphthyl-2,2′-diyl hydrogenphosphate.e 2a (4 equiv.), and ZnMe2 (4 equiv.).f 2a (5 equiv.), and ZnMe2 (4 equiv.). 1.c Yield of isolated product.d (R)-(−)-1,1′-Binaphthyl-2,2′-diyl hydrogenphosphate.e 2a (4 equiv.), and ZnMe2 (4 equiv.).f 2a (5 equiv.), and ZnMe2 (4 equiv.). |
||||
| 1 | BiCl3 | Toluene | 40 | Trace |
| 2 | ZnCl2·Et2O | Toluene | 40 | 7 |
| 3 | CF3CO2H | Toluene | 40 | Trace |
| 4 | MSA | Toluene | 40 | 7 |
| 5 | (R)-(−)-BPAd | Toluene | 40 | 11 |
| 6 | p-TsOH | Toluene | 40 | 18 |
| 7 | CSA | Toluene | 40 | 32 |
| 8 | CH3CO2H | Toluene | 40 | 41 |
| 9 | HCO2H | Toluene | 40 | 43 |
| 10e | HCO2H | Toluene | 40 | 39 |
| 11f | HCO2H | Toluene | 40 | 32 |
| 12 | HCO2H | THF | 40 | Trace |
| 13 | HCO2H | Hexane | 40 | 30 |
| 14 | HCO2H | CH2Cl2 | 40 | 25 |
| 15 | HCO2H | CH2Cl2 | r.t. | 46 |
On the basis of this initial observation, we used 5-methoxysalicylaldehyde (1a), 3-methoxyprop-1-yne (2a), and 6,6-dimethyl-1-vinylcyclohex-1-ene (3a) as model substrates for further optimization (Table 1). Various acids were initially evaluated in toluene at 40 °C (entries 1–9). The experimental results indicated that HCO2H is a beneficial acid. By using the ideal acid, we discovered that the stoichiometric ratio of 2a and ZnMe2 in alkynylation sequence effectively facilitated the desired transformation (entries 9–11). We then screened several organic solvents, including THF, hexane and CH2Cl2 (entries 12–15). As a result, CH2Cl2 was found to be the best choice at room temperature, and the anticipated spirocycle containing product 4a can be produced in 46% yield. We also performed a stepwise investigation under the optimal conditions but leading to a lower yield (22%, for details see ESI†). So we proposed that the existence of ZnMe2 may play a very significant role in the one-pot process.
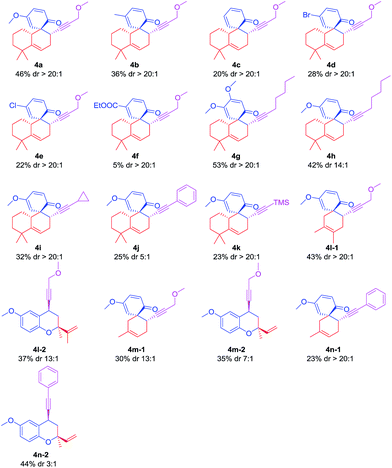
Having identified optimal reaction conditions (Table 1, entry 15), we first examined the substrate scope of this transformation with respect to the substituted salicylaldehydes partner. Table 2 shows that the electronic effect of the substrates is very significant. Electron-donating groups, such as dimethoxy (4g), methoxy (4a, h), and methyl (4b) are more favorable for the envisioned spirocycle products than the electron-withdrawing groups, such as bromo (4d), chloro (4e), and ester (4f). The reaction is also restricted to the terminal alkyne (4a, 4h–k). The steric effect of these electron-rich alkynes exerts a great impact on the yields (23–46%). Functionalized 1,3-butadienes were also investigated. We obtained both spirocycle products (4l-1, 4m-1, 4n-1) and benzopyran compounds (4l-2, 4m-2, 4n-2) when the reaction was treated with isoprene or 2,3-dimethyl-1,3-butadiene. Benzopyran compounds were not observed when using 6,6-dimethyl-1-vinylcyclohex-1-ene (3a) as diene (Table 2, 4a–k). We then speculated that the benzopyran products corresponding to 3a are unstable under acidic conditions.
a For all compounds, dr was determined by 1H NMR spectroscopy, if diastereoisomers could not be detected in 1H NMR spectra, we identified the dr as >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 4g and 4i were also analysed by HPLC (see ESI). 1. 4g and 4i were also analysed by HPLC (see ESI). |
|---|
 |
Based on these results and our previous findings, a plausible reaction mechanism for this spirocyclization is illustrated in Scheme 2. The reaction of ZnMe2 with terminal alkyne (2) generated an active alkynylzinc,20 which attacked the substituted salicylaldehyde (1) to form alcohol (5). Under acidic conditions, 5 was converted into carbocation TS-A, which was difunctionally stabilized by a phenyl group and an acetylenyl group through conjugation effects. The formed carbocation TS-A underwent electrophilic addition with diene 3 to deliver the resonance hybrids TS-B and TS-C. TS-C followed by enol–keto tautomerization of the phenol ring to produce spirocycle 4-1 (path a). At the same time, TS-B underwent intramolecular nucleophilic addition to form the benzopyran compound 4-2 through path b.
Conclusions
We developed an approach to construct spiro[5.5]undecanes. The ZnMe2-promoted alkynylation of salicylaldehyde and HCO2H-mediated dearomatizative cyclization occurred in sequence in a one-pot process, which not only involves readily available starting materials but also demonstrates high diastereoselectivities. Utilizing multicomponent synthesis and one-pot process as the core strategies, a number of spirocycles were prepared smoothly.Acknowledgements
The authors acknowledge the financial support provided by the National Natural Science Foundation of China (21202069, 21272099, and 21472078). We thank Dr Yuan Zhang and Prof. Zhi-Xiang Xie (Lanzhou University) for helpful discussion.Notes and references
- G. L. Plourde, Reviews on spirocycle compounds: a special issue “Spiro Compounds”, Molecules Search PubMed.
- (a) J. J. Sims, G. H. Y. Lin and R. M. Wing, Tetrahedron Lett., 1974, 15, 3487 CrossRef; (b) D. J. Kennedy, I. A. Selby and R. H. Thomson, Phytochemistry, 1988, 27, 1761 CrossRef CAS; (c) Y. Asakawa, T. Motoo, T. Masuya and J.-P. Frahm, Phytochemistry, 1990, 29, 1577 CrossRef CAS; (d) E. C. D. C. Araújo, M. A. S. Lima and E. R. Silveira, Magn. Reson. Chem., 2004, 42, 1049 CrossRef PubMed; (e) K. M. Brundret, W. Dalziel, B. Hesp, J. A. J. Jarvis and S. Neidle, J. Chem. Soc., Chem. Commun., 1972, 1027 RSC.
- (a) C. S. Vairappan, M. Daitoh, M. Suzuki, T. Abe and M. Masuda, Phytochemistry, 2001, 58, 291 CrossRef CAS PubMed; (b) C. S. Vairappan, Biomol. Eng., 2003, 20, 255 CrossRef CAS PubMed; (c) HeLa: IC50 = 4.1 mM (lag phase), 1.3 mM (log phase); Hep-2: IC50 = 2.4 mM (lag phase), 2.0 mM (log phase); T. Dias, I. Brito, L. Moujir, N. Paiz, J. Darias and M. Cueto, J. Nat. Prod., 2005, 68, 1677 CrossRef CAS PubMed.
- (a) R. A. Buchnall, H. Moores, R. Simms and B. Hesp, Antimicrob. Agents Chemother., 1973, 4, 294 CrossRef; (b) S. Ikegami, T. Taguchi, M. Ohashi, M. Oguro and Y. Mano, Nature, 1978, 275, 458 CrossRef CAS PubMed.
- (a) L. Pouységu, S. Chassaing, D. Dejugnac, A.-M. Lamidey, K. Miqueu, J.-M. Sotiropoulos and S. Quideau, Angew. Chem., Int. Ed., 2008, 47, 3552 CrossRef PubMed; (b) T. Dohi, A. Maruyama, N. Takenaga, K. Senami, Y. Minamitsuji, H. Fujioka, S. B. Caemmerer and Y. Kita, Angew. Chem., Int. Ed., 2008, 47, 3787 CrossRef CAS PubMed; (c) T. Dohi, N. Takenaga, T. Nakae, Y. Toyoda, M. Yamasaki, M. Shiro, H. Fujioka, A. Maruyama and Y. Kita, J. Am. Chem. Soc., 2013, 135, 4558 CrossRef CAS PubMed; (d) M. Uyanik, T. Yasui and K. Ishihara, Angew. Chem., Int. Ed., 2010, 49, 2175 CrossRef CAS PubMed; (e) M. Uyanik, T. Yasui and K. Ishihara, Angew. Chem., Int. Ed., 2013, 52, 9215 CrossRef CAS PubMed; (f) M. Uyanik, N. Sasakura, E. Kaneko, K. Ohori and K. Ishihara, Chem. Lett., 2015, 44, 179 CrossRef; (g) M. Uyanik, N. Sasakura, M. Kuwahata, Y. Ejima and K. Ishihara, Chem. Lett., 2015, 44, 381 CrossRef CAS.
- (a) J. X. Liang, J. B. Chen, F. X. Du, X. H. Zeng, L. Li and H. B. Zhang, Org. Lett., 2009, 11, 2820 CrossRef CAS PubMed; (b) H. Liang and M. A. Ciufolini, Chem.–Eur. J., 2010, 16, 13262 CrossRef CAS PubMed; (c) C. Zheng, L. Wang, J. J. Li, L. F. Wang and D. Z. G. Wang, Org. Lett., 2013, 15, 4046 CrossRef CAS PubMed; (d) A. M. Harned, Tetrahedron Lett., 2014, 55, 4681 CrossRef CAS PubMed; (e) H. Abdellaoui and J. X. Xu, Tetrahedron, 2014, 70, 4323 CrossRef CAS; (f) D.-Y. Zhang, L. Xu, H. Wu and L.-Z. Gong, Chem.–Eur. J., 2015, 21, 10314 CrossRef CAS PubMed; (g) J.-C. Andrez, M.-A. Giroux, J. Lucien and S. Canesi, Org. Lett., 2010, 12, 4368 CrossRef CAS PubMed.
- (a) T. Nemoto, Y. Ishige, M. Yoshida, Y. Kohno, M. Kanematsu and Y. Hamada, Org. Lett., 2010, 12, 5020 CrossRef CAS PubMed; (b) Q.-F. Wu, W.-B. Liu, C.-X. Zhuo, Z.-Q. Rong, K.-Y. Ye and S.-L. You, Angew. Chem., Int. Ed., 2011, 50, 4455 CrossRef CAS PubMed; (c) S. Rousseaux, J. G. Fortanet, M. A. D. A. Sanchez and S. L. Buchwald, J. Am. Chem. Soc., 2011, 133, 9282 CrossRef CAS PubMed.
- A. Rudolph, P. H. Bos, A. Meetsma, A. J. Minnaard and B. L. Feringa, Angew. Chem., Int. Ed., 2011, 50, 5834 CrossRef CAS PubMed.
- T. Oguma and T. Katsuki, Chem. Commun., 2014, 50, 5053 RSC.
- (a) J. Nan, Z. J. Zuo, L. Luo, L. Bai, H. Y. Zheng, Y. N. Yuan, J. J. Liu, X. J. Luan and Y. Y. Wang, J. Am. Chem. Soc., 2013, 135, 17306 CrossRef CAS PubMed; (b) Z. J. Zuo, X. Yang, J. J. Liu, J. Nan, L. Bai, Y. Y. Wang and X. Luan, J. Org. Chem., 2015, 80, 3349 CrossRef CAS PubMed; (c) J. Zheng, S.-B. Wang, C. Zheng and S.-L. You, J. Am. Chem. Soc., 2015, 137, 4880 CrossRef CAS PubMed.
- (a) A. Seoane, N. Casanova, N. Quiñones, J. L. Mascareñas and M. Gulías, J. Am. Chem. Soc., 2014, 136, 7607 CrossRef CAS PubMed; (b) S. Kujawa, D. Best, D. J. Burns and H. W. Lam, Chem.–Eur. J., 2014, 20, 8599 CrossRef CAS PubMed.
- S. L. Gu, L. Luo, J. J. Liu, L. Bai, H. Y. Zheng, Y. Y. Wang and X. J. Luan, Org. Lett., 2014, 16, 6132 CrossRef CAS PubMed.
- H. Y. Zheng, L. Bai, J. J. Liu, J. Nan, Z. J. Zuo, L. Yang, Y. Y. Wang and X. J. Luan, Chem. Commun., 2015, 51, 3061 RSC.
- B. S. Matsuura, A. G. Condie, R. C. Buff, G. J. Karahalis and C. R. J. Stephenson, Org. Lett., 2011, 13, 6320 CrossRef CAS PubMed.
- J. C. Green and T. R. R. Pettus, J. Am. Chem. Soc., 2011, 133, 1603 CrossRef CAS PubMed.
- E. E. Podlesny, P. J. Carroll and M. C. Kozlowski, Org. Lett., 2012, 14, 4862 CrossRef CAS PubMed.
- Y. S. Rozhkova, K. A. Galata, A. A. Gorbunov, Y. V. Shklyaev, M. A. Ezhikova and M. I. Kodess, Synlett, 2014, 25, 2617 CrossRef CAS.
- G. F. Han, Y. X. Liu and Q. M. Wang, Org. Lett., 2014, 16, 3188 CrossRef CAS PubMed.
- J. D. Shao, L. Q. Li, J. Zhang, T. Tian, J. J. Xue, Y. Zhang and Y. Li, Synlett, 2015, 26, 827 CrossRef CAS.
- L. Pu and H.-B. Yu, Chem. Rev., 2001, 101, 757 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1447716. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra02463g |
| This journal is © The Royal Society of Chemistry 2016 |






