Multi-modal imaging of HeLa cells using a luminescent ZnS:Mn/NaGdF4:Yb:Er nanocomposite with enhanced upconversion red emission
Abstract
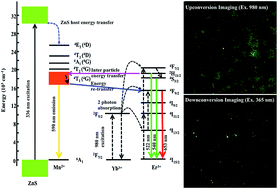
The multi-modal luminescence properties of the ZnS:Mn/NaGdF4:Yb:Er nanocomposite were investigated. The ZnS:Mn/NaGdF4:Yb:Er nanocomposite was synthesized using a single-step hot injection method. Its crystallinity was confirmed using X-ray diffraction, and was compared with the crystallinities of NaGdF4:Yb:Er nanoparticles and ZnS:Mn quantum dots synthesized by thermal decomposition. Composite formation and morphology changes were investigated by transmission electron microscopy. Enhanced upconversion red emission (654 nm, 4F9/2–4I15/2) was observed for NaGdF4:Yb:Er in the presence of ZnS:Mn. Analysis of the excitation and downconversion emission spectra of ZnS:Mn/NaGdF4:Yb:Er indicated modified emission at lower wavelengths. Cathodoluminescence measurements indicated that the ZnS:Mn/NaGdF4:Yb:Er nanocomposite had a broad visible emission from green to red wavelengths. Cytotoxicity and stability measurements were executed to ensure the toxicity of the synthesized composite and their luminescence stability in water as well as phosphate buffered solution. The nanocomposite had multi-modal characteristics, and was used in the upconversion and downconversion optical imaging of living He–La cells. The multi-modal luminescence capability of the nanocomposite was suitable for upconversion and downconversion imaging.

 Please wait while we load your content...
Please wait while we load your content...