Towards the understanding of non-thermal air plasma action: effects on bacteria and fibroblasts†
Abstract
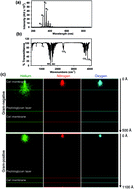
Non-thermal plasma research has put a growing focus on the bacteria inactivation problem. In this article we show how low temperature atmospheric plasma destroys Gram-positive and Gram-negative bacteria and discuss the mechanisms of plasma bactericidal effects and a discrepancy in the plasma-triggered effects and ozone (which is a component of air plasma gases). The proven safety of air plasma for fibroblasts is a key factor for the medical applications of plasma.

 Please wait while we load your content...
Please wait while we load your content...