Stable and magnetically reusable nanoporous magnetite micro/nanospheres for rapid extraction of carcinogenic contaminants from water†
Abstract
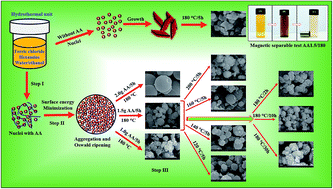
We report the growth of porous magnetite microspheres via a template-free hydrothermal route at relatively low temperatures. The growth parameters including the concentration of reactant, reaction temperature and reaction time were examined for the formation of nanopores in the microspheres. Ascorbic acid played a crucial role in the formation of nanoporous microspheres, and also acts as a reducing agent, resulting from Fe3+ conversion into Fe2+ iron during the hydrothermal process. The resulting samples were used to remove acid orange 7 (AO7) and Cr(VI) from water at room temperature via adsorption. The maximum AO7 adsorption capacity calculated from the Langmuir–Freundlich model was found to be 69.13 mg g−1 for an optimum porous structure, which is significantly greater than that of structures formed without ascorbic acid (i.e., 4.61 mg g−1). Simultaneously, the Cr(VI) removal performance of the porous structure (i.e., 96.98 mg g−1) was found to be superior than that of pristine structure (i.e., 10.67 mg g−1). The enhanced removal activity was attributed to the existence of a large number of nanopores, resulting in a large specific surface area of 87.36 m2 g−1. Recycling tests were carried using a magnetic field to separate the powder samples from water, and the samples were found to be stable for up to three cycles. These results show that these porous microspheres have potential applications in cost-effective wastewater treatment.

 Please wait while we load your content...
Please wait while we load your content...