Intermolecular ionic cross-linked sulfonated poly(ether ether ketone) membranes with excellent mechanical properties and selectivity for direct methanol fuel cells
Guibin Lia,
Chengji Zhao*ab,
Ying Cuia,
Tao Ronga,
Chongyi Zhua and
Hui Naab
aAlan G. MacDiarmid Institute, College of Chemistry, Jilin University, Changchun 130012, PR China. E-mail: zhaochengji@jlu.edu.cn; Fax: +86 431 85168870; Tel: +86 431 85168870
bKey Laboratory of Advanced Batteries Physics and Technology (Ministry of Education), Jilin University, Changchun 130012, PR China
First published on 22nd February 2016
Abstract
Amino-substituted poly(ether ether ketone) (APEEK) and sulfonated poly(ether ether ketone) (SPEEK, IEC = 2.07 mequiv. g−1) have been synthesized via nucleophilic aromatic substitution reaction. The structures of APEEK and SPEEK were characterized by 1H NMR spectra. The composite membranes based on APEEK and SPEEK were confirmed by their FTIR spectra, indicating the formation of intermolecular ionic cross-linking networks between amino and sulfonic groups. The water uptake, proton and methanol transport properties of composite membranes were also determined for fuel cell applications. The results showed that the composite membranes exhibit high selectivity, appropriate proton conductivities as well as reduced water uptake and methanol permeability when compared with the pristine SPEEK membrane. Furthermore, it should be noted that the intermolecular ionic cross-linking effectively improved the tensile strength, breaking elongation, and thermal stabilities of the membranes. In particular, the SPEEK-10 membrane (the weight ratio of APEEK is 10%) showed a tensile strength of 121.2 MPa and breaking elongation of 93.5%, which were 1.5 times and 2.5 times higher than those of pristine SPEEK, respectively. The high selectivity, thermal and mechanical properties indicate that the composite membranes are promising to be used as proton exchange membranes for direct methanol fuel cells.
Introduction
Polymer electrolyte membrane fuel cells (PEMFCs), especially direct methanol fuel cells (DMFCs), have recently received considerable attention as clean and efficient power sources for automotive, stationary, and portable applications due to their high energy density, conversion efficiency, convenient fuel supply, quick start times and zero emission levels.1–4 To optimize the efficiency of the PEMFCs, high demands are put on the proton exchange membrane (PEM), such as high conductivity, excellent methanol resistance properties and good mechanical properties. Currently, a series of membranes which were made of perfluorinated sulfonic acid copolymers, such as Nafion (Dupont), are the commercial PEM materials for DMFCs because of their high proton conductivities, excellent mechanical and chemical properties.5 However, their limited operating temperature range (<80 °C), high methanol/gas diffusion, environmental recyclability, and high cost are perceived as significant disadvantages. Recently, aromatic hydrocarbon polymers have been developed as alternative PEM materials.6–8 The most widely reported aromatic PEMs materials, such as sulfonated derivatives of poly(ether ether ketone) (SPEEK),9,10 poly(arylene ether sulfone),11,12 polyethersulfone,13,14 and polybenzimidazoles,15,16 are a class of high-performance engineering thermoplastic materials.Generally, membranes based on these sulfonated aromatic polymers only reach high proton conductivity at high IEC values with excessive water uptake. However, high IEC (ion exchange capacity) values usually lead to high methanol permeability and large dimensional variations as well as inadequate mechanical properties; all these disadvantages would render the membranes unsuitable for application in PEMFCs. To overcome the problem, several methods have been developed, such as cross-linking,17,18 acid–base composite19,20 and layer-by-layer modification.21,22 Among these modified membranes, the composite membranes are potential alternatives due to their easy preparation without any additional treatments. Different results showed that the incorporation of imidazole, benzimidazole or amino groups into sulfonated polymers could bring many excellent properties, such as reduced water uptake, dimensional stability and methanol resistance property.23 Muthumeenal et al. reported a novel composite PEM based on sulfonated polyethersulfone and N-phthaloyl chitosan. Compared to Nafion 117, the composite membranes showed low methanol permeability and better electrochemical selectivity.24 Erkartal et al. reported a poly(vinyl alcohol)/poly(2-acrylamido-2-methylpropane sulfonic acid) (PAMPS)/zeolitic imidazolate framework (ZIF-8) homogenous composite membranes. The addition of ZIF-8 was beneficial to the proton conduction by forming hydrogen bonds and the proton conductivity could reach up to 0.134 S cm−1 with the highest PAMPS content.25 Li et al. reported a novel benzimidazole-grafted poly(ether ether ketone) (PEEK-BI) and the composite membranes based on SPEEK and PEEK-BI. The composite membrane showed high selectivity, improved mechanical properties and appropriate proton conductivities.26 Our groups had prepared the composite membranes by blending SPEEK and polybenzimidazole, which exhibited moderate swelling properties and low methanol permeability.27 Although the tensile strength of composite membrane was enhanced, the elongation at a break decreased to 13%, which is relative low for practical application in DMFCs. Therefore, it is still a big challenge to obtain the composite membranes with moderate proton conductivity, fuel resistance, dimensional stability and mechanical properties.
In this study, we synthesized a sulfonated poly(ether ether ketone) and a novel amino-substituted poly(ether ether ketone) (APEEK). The aim of this study is to develop the composite membranes based on SPEEK and APEEK. We used the 1H NMR to confirm the structure of SPEEK and APEEK. Both of the two polymers have similar main-chain structure and can be easily dissolved in dimethylsulfoxide (DMSO), which may result in a good compatibility. The interaction between amino groups of APEEK and sulfonic acid groups of SPEEK leads to the formation of intermolecular ionic cross-linking networks, which was confirmed by their FTIR spectra. Moreover, due to the intermolecular ionic cross-linking networks, the water uptake and methanol permeability was reduced, thus resulting in a high selectivity. Most evidently, a remarkably increase in the mechanical property was observed for these composite membranes, including the tensile strength and the breaking elongation. The ion exchange capacity, thermal stability and proton conductivity of all prepared membranes were also investigated in detail.
Experimental
Materials
4,4′-Difluoro diphenylmethanone (DFB), bisphenol A (BPA), hydrochloric acid (5 mol L−1) and 2,2′-bis(3-amino-4-hydroxyphenyl)propane (ami-BPA) were purchased from Sigma-Aldrich. DMSO, tetramethylene sulfone (TMS), acetone and anhydrous potassium carbonate were purchase from TCI chemical company. Sulfonated 4,4′-difluorodiphenylmethanone (SDFB) and 3,3′,5,5′-tetramethyl-4,4′-dihydroxybiphenyl (TMBP) were synthesized according to our previous work.28 All other solvents and reagents were reagent grade and were used as received.Synthesis of amino-substituted poly(ether ether ketone) (APEEK)
The synthetic procedure of APEEK copolymer (ami-BPA/BPA = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) is described as follows in Scheme 1(a). Samples of ami-BPA (5.17 g, 20 mmol), BPA (4.56 g, 20 mmol), DFB (8.73 g, 40 mmol), K2CO3 (6.68 g, 44 mmol), TMS (50 mL) and toluene (25 mL) were added into a 250 mL three-neck flask equipped with a mechanical stirrer, a Dean–Stark trap, and a nitrogen inlet/outlet. The solution was allowed to reflux at 130 °C, while the water was azeotropically removed from the reaction mixture. After 3 h, the toluene was removed from the reaction by slowly increasing the temperature to 170 °C, and then the reaction was allowed to continue for another 2–3 h. When the viscosity was observed to increase dramatically, the mixture was slowly poured into 1000 mL deionized water. The resulting fibrous copolymer was washed with hot water several times and dried under vacuum at 80 °C for 24 h to give APEEK.
50) is described as follows in Scheme 1(a). Samples of ami-BPA (5.17 g, 20 mmol), BPA (4.56 g, 20 mmol), DFB (8.73 g, 40 mmol), K2CO3 (6.68 g, 44 mmol), TMS (50 mL) and toluene (25 mL) were added into a 250 mL three-neck flask equipped with a mechanical stirrer, a Dean–Stark trap, and a nitrogen inlet/outlet. The solution was allowed to reflux at 130 °C, while the water was azeotropically removed from the reaction mixture. After 3 h, the toluene was removed from the reaction by slowly increasing the temperature to 170 °C, and then the reaction was allowed to continue for another 2–3 h. When the viscosity was observed to increase dramatically, the mixture was slowly poured into 1000 mL deionized water. The resulting fibrous copolymer was washed with hot water several times and dried under vacuum at 80 °C for 24 h to give APEEK.
Synthesis of sulfonated poly(ether ether ketone) (SPEEK)
The preparation of SPEEK copolymer (SDFB/DFB = 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) is described as follows in Scheme 1(b). Samples of SDFB (7.60 g, 18 mmol), DFB(2.62 g, 12 mmol), TMBP (7.26 g, 30 mmol), K2CO3 (4.56 g, 33 mmol), TMS (40 mL) and toluene (18 mL) were added into a 250 mL three-neck flask equipped with a mechanical stirrer, a Dean–Stark trap, and a nitrogen inlet/outlet. The solution was allowed to reflux at 130 °C, while the water was azeotropically removed from the reaction mixture. After 4 h, the toluene was removed from the reaction by slowly increasing the temperature to 210 °C, and then the reaction was allowed to continue for another 5–6 h. When the viscosity was observed to increase dramatically, the mixture was slowly poured into 500 mL acetone. The resulting fibrous copolymer was washed with acetone two times, then hot water several times and dried under vacuum at 80 °C for 24 h to give SPEEK.
40) is described as follows in Scheme 1(b). Samples of SDFB (7.60 g, 18 mmol), DFB(2.62 g, 12 mmol), TMBP (7.26 g, 30 mmol), K2CO3 (4.56 g, 33 mmol), TMS (40 mL) and toluene (18 mL) were added into a 250 mL three-neck flask equipped with a mechanical stirrer, a Dean–Stark trap, and a nitrogen inlet/outlet. The solution was allowed to reflux at 130 °C, while the water was azeotropically removed from the reaction mixture. After 4 h, the toluene was removed from the reaction by slowly increasing the temperature to 210 °C, and then the reaction was allowed to continue for another 5–6 h. When the viscosity was observed to increase dramatically, the mixture was slowly poured into 500 mL acetone. The resulting fibrous copolymer was washed with acetone two times, then hot water several times and dried under vacuum at 80 °C for 24 h to give SPEEK.
Preparation of composite membranes
The procedure for preparing SPEEK/APEEK composite membranes is shown in Scheme 2. The fixed amount of SPEEK in sodium form and APEEK (the weight ratio is listed Table 1) were dissolved in 10 mL of DMSO to get mixed solutions with different weight ratios of APEEK. The mixed copolymer solutions were then cast onto glass plates and dried at 60 °C for 10 h. To remove any excess of the solvent, the membranes were dried under vacuum at 80 °C for 24 h. The acidification of the composite membranes was performed by immersing the membranes into 4 M HCl solution for 24 h and washed with deionized water to remove residual acid. The obtained composite membranes in acid form were then dried in vacuum at 80 °C for 12 h. We denote the composite membrane as SPEEK-xx (xx: the weight percentage of APEEK in the composite membrane), whose thicknesses are in the range of 70–90 μm.| Sample name | APEEK content | T5%a (°C) | Young's modulus (MPa) | Maximum elongation (%) | Tensile strength (MPa) |
|---|---|---|---|---|---|
| a T5% (°C): 5% weight loss temperature in TGA test. | |||||
| SPEEK | 0% APEEK | 308.3 | 1281.4 | 37.9 | 78.6 |
| SPEEK-1 | 1% APEEK | 316.1 | 1298.2 | 39.4 | 92.4 |
| SPEEK-2.5 | 2.5% APEEK | 318.4 | 1427.6 | 51.6 | 94.6 |
| SPEEK-5 | 5% APEEK | 319.1 | 1706.4 | 86.1 | 108.6 |
| SPEEK-10 | 10% APEEK | 320.2 | 1994.1 | 93.5 | 121.2 |
Characterization of composite membranes
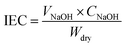 | (1) |
 | (2) |
The swelling ratio was calculated from the change of membrane in length and thickness by:
 | (3) |
 | (4) |
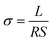 | (5) |
 | (6) |
Results and discussion
Synthesis of copolymers and characterization of composite membranes
APEEK and SPEEK were prepared by the aromatic nucleophilic substitution condensation in a TMS/toluene solvent system. The intrinsic viscosity (ηsp/c) was measured from polymer solution concentration of 0.5 g dL−1 in DMSO at 25 °C. The viscosity of APEEK and SPEEK is 1.21 and 1.10 dL g−1, respectively, indicating high molecular weight of the resulting copolymers.The chemical structure of SPEEK was confirmed by 1H NMR spectroscopy in DMSO-d6. As shown in Fig. 1, the signal at 8.2 ppm was assigned to the aromatic protons adjacent to the sulfonic acid groups, while the signal at 7.8 ppm was assigned to the aromatic protons adjacent to the carbonyl groups. These characteristic proton resonances in the 1H NMR spectrum indicated that SPEEK was successfully synthesized.
As shown in Scheme 1, APEEK containing two amino groups in each repeat unit was synthesized by the polycondensation of difluoro-monomer (DFB) and two bisphenol monomers (ami-BPA/BPA = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). The polymerization reactions took place smoothly as no evident cross-linking was found even at above 180 °C. Fig. 2 provides the representative 1H NMR spectrum of APEEK. The proton peaks at 4.89 ppm attributed to the hydrogen atom of –NH2 groups, and the proton peaks for the protons of –CH3 groups were observed about 1.53 ppm. The results indicated that the APEEK was completely synthesized.
5). The polymerization reactions took place smoothly as no evident cross-linking was found even at above 180 °C. Fig. 2 provides the representative 1H NMR spectrum of APEEK. The proton peaks at 4.89 ppm attributed to the hydrogen atom of –NH2 groups, and the proton peaks for the protons of –CH3 groups were observed about 1.53 ppm. The results indicated that the APEEK was completely synthesized.
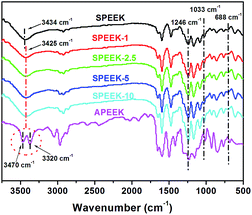
Fig. 3 shows the FTIR spectra of SPEEK, APEEK and SPEEK-xx composite membranes. The observed bands at 3320 cm−1 and 3470 cm−1 were assigned to the symmetric and asymmetric stretching vibrations of –NH2 of APEEK. As for the pristine SPEEK membrane, the observed bands at 1246 and 1033 cm−1 were assigned to the symmetric and asymmetric stretching vibrations of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The infrared band at about 688 cm−1 can be assigned to the S–O stretching of sulfonated groups. The 3434 cm−1 for the SPEEK corresponded to the hydroxyl groups in water that interact with sulfonic acid group of the polymer less than 100% RH. These characteristic bands of sulfonated groups were also observed in the SPEEK-xx composite membranes. However, the stretching infrared band located at 3320 cm−1 and 3470 cm−1 for –NH2 groups of APEEK completely disappeared in the spectra of SPEEK-xx composite membrane.
O. The infrared band at about 688 cm−1 can be assigned to the S–O stretching of sulfonated groups. The 3434 cm−1 for the SPEEK corresponded to the hydroxyl groups in water that interact with sulfonic acid group of the polymer less than 100% RH. These characteristic bands of sulfonated groups were also observed in the SPEEK-xx composite membranes. However, the stretching infrared band located at 3320 cm−1 and 3470 cm−1 for –NH2 groups of APEEK completely disappeared in the spectra of SPEEK-xx composite membrane.
At the same time, a new infrared band was observed at 3425 cm−1 in SPEEK-xx membranes, which is located between the 3434 cm−1 of SPEEK and 3420 cm−1 of APEEK. This might be caused by the interaction between the sulfonic acid groups of SPEEK and the amino groups of APEEK.
The thermal and mechanical properties
As shown in Fig. 4, SPEEK showed a classical two-step thermal decomposition curve, which is similar to those reported sulfonated poly(arylene ether ketone).29 The initiative weight loss occurred at about 270 °C, which was associated with the elimination of the sulfonic acid groups. The last weight loss with the onset temperature around 450 °C corresponded to the degradation of main backbone. All the composite membranes showed improved thermal stability when compared to the pristine SPEEK. The pristine SPEEK membrane displayed the 5% weight loss temperature (T5%) of 308.3 °C, while the composite membranes possessed the T5% values in the range of 316–320 °C. It is well-known that the sulfonic acid groups can form ionic-bonds with the basic amino groups, thus leading to the stabilization of the aromatic sulfonic acid groups. The formed intermolecular ionic cross-linked network is beneficial to improving the thermal stability of composite membranes.24,27The mechanical properties of membranes were evaluated and the results are presented in Fig. 5 and Table 1. The pristine SPEEK showed a Young's modulus of 1281.4 MPa, an elongation at break of 39.4%, and a tensile strength at maximum load of 78.6 MPa. All the composite membranes displayed the greatly improved Young's modulus in the range of 1298.2–1994.1 MPa and the tensile strength in the range of 92.4–121.2 MPa. Especially, the composite membranes also showed much enhanced elongation at break ranging from 39.4% to 93.5%, which is much better than that of pristine SPEEK membrane. Considering the tensile strength and the breaking elongation of composite membranes, they were both increased with the increasing of the addition of APEEK in the composite. It is assumed that the interaction between the sulfonic acid and the amino groups restricts the molecular motion of the polymer chains and results in the much tough and ductile membranes. The similar phenomenon was observed by Guo et al. and Li et al.26,30,31
Membrane properties: IEC, water uptake, and swelling ratio
Table 2 summaries the IEC, water uptake, and swelling ratio of SPEEK-xx composite membranes. IEC represents the amount of exchangeable protons in ionomer membranes. The IEC of membranes was determined by an acid–base titration method, which was commonly used to determine the IEC of acid functionalized membranes and the measured IEC values of SPEEK-xx are in the range of 1.43–1.73 mequiv. g−1 as shown in Table 2. With the content of APEEK increasing, the composite membranes exhibited the reduced IEC values. The difference in IEC between the pristine SPEEK and SPEEK-xx composite membranes can be attributed to the introduction of APEEK, which diluted the density of sulfonic acid groups in the membranes. On the other hand, the formation of intermolecular ionic cross-linking between amino and sulfonic acid groups stabilizes part of H+ in sulfonic groups and makes them cannot be exchanged by Na+ in large excess NaCl solution.19| Membranes | IEC (mequiv. g−1) | Proton conductivity (mS cm−1) | Water uptake (%) | Swelling ratio (%) | Methanol permeability (10−7 cm2 s−1) | |||
|---|---|---|---|---|---|---|---|---|
| In thickness (Δd) | In plane (Δl) | |||||||
| 25 °C | 80 °C | 25 °C | 80 °C | 80 °C | 80 °C | |||
| SPEEK | 1.73 | 41.1 | 122.8 | 45.6 | 59.6 | 31.7 | 15.9 | 16.92 |
| SPEEK-1 | 1.70 | 38.9 | 120.3 | 43.2 | 53.5 | 24.6 | 14.2 | 15.12 |
| SPEEK-2.5 | 1.66 | 33.4 | 108.2 | 38.6 | 49.4 | 13.9 | 11.8 | 12.91 |
| SPEEK-5 | 1.57 | 22.3 | 91.7 | 35.7 | 43.2 | 9.6 | 9.2 | 9.12 |
| SPEEK-10 | 1.43 | 17.2 | 82.4 | 25.1 | 31.6 | 6.3 | 6.8 | 4.47 |
Water uptake is an important parameter for PEMs because of its critical influence on the proton conductivity, dimensional stability and membrane-electrode compatibility. According to the “vehicle mechanism” of proton conduction, protons within PEM could not be conducted unless they are hydrated by water.31,32 However, excessive water uptake would deteriorate the dimensional stability, which leads to the loss of mechanical properties. So the unification of high proton conductivity, low water uptake and dimensional change is desired for PEM applications. The water uptake and swelling ratio of SPEEK-xx membranes were evaluated by comparing their hydrated state with dry state membranes at different temperatures.
As shown in Fig. 6 and Table 2, the pristine SPEEK membrane exhibited the water uptake of 45.6% at 25 °C and 59.6% at 80 °C. The composite membranes showed a greatly reduced water uptake with the content of APEEK increasing. For example, SPEEK-10 with the highest content of APEEK had the lowest water uptake of 31.6% at 80 °C. Moreover, the swelling ratio of the composite membranes (Fig. 7) in length and thickness also showed greatly improved dimensional stability when compared to the pristine SPEEK. This is beneficial to the application of membranes in PEMFCs. The reduced water uptake and improved dimensional stability were attributed to the reduced IEC values and the interaction between amino and sulfonic groups, which restricts the movement of polymer chain.
Proton conductivity and methanol permeability
The performance of a fuel cell is usually based on the proton conductivity of the PEM material. In this work, proton conductivity of membranes was measured in 100% relative humidity by AC impedance spectroscopy. As shown in Table 2, the IEC values of SPEEK-xx changed from 1.43 to 1.73 mequiv. g−1 and the proton conductivity increased from 82.4 to 122.8 mS cm−1 at 80 °C. As shown in Fig. 8, the proton conductivity of all the membranes increased as a function of temperature and IEC. For example, the pristine SPEEK had the highest proton conductivity of 122.8 mS cm−1 at 80 °C because it possessed the highest IEC value and water uptake. The composite membranes exhibited the reduced proton conductivity when compared with the pristine SPEEK. However, the proton conductivity of all composite membranes was still above 80 mS cm−1 at 80 °C, which meets the requirements of DMFCs.33 The reduced proton conductivity can be attributed to the reduction of water uptake, which restricts the formation of continuous proton transferring channels. Besides, the interaction between amino and sulfonic acid groups makes part of H+ in sulfonic acid be fixed which is useless for proton conducting.Apart from high proton conductivity, the PEMs in the DMFCs must possess excellent methanol resistance property as well. The methanol permeability of SPEEK-xx membranes is shown in Table 2, and it is ranged from 4.47 × 10−7 cm2 s−1 to 15.12 × 10−7 cm2 s−1, which are lower than the value of the pristine SPEEK (16.92 × 10−7 cm2 s−1). In order to explore the comprehensive performance of the proton conductivity and the methanol resistance, we adopt the concept of selectivity (the ratio of conductivity to methanol permeability) for evaluating membrane performance. Normally, a high selectivity often means a good performance of the battery. Fig. 9 shows the relative selectivity of the SPEEK-xx membranes and the pristine SPEEK. As shown in Fig. 8, the proton conductivity showed a slow reduction with the increasing of the content of APEEK, while the methanol permeability showed a rapid decline with the content of APEEK increasing. The results induced the improved selectivity of the composite membranes. As the content of APEEK increasing from 1% to 10%, the relative selectivity of SPEEK-xx composite membranes increased from 7.96 × 105 S s cm−3 to 18.44 × 105 S s cm−3. They were much higher than that of the pristine SPEEK (7.26 × 105 S s cm−3), indicating that the SPEEK/APEEK composite membranes could be potentially used as PEMs in DMFCs.
Conclusions
In summary, a novel amino-substituted and sulfonated poly(ether ether ketone) were successfully synthesized, respectively. The composite membranes based on SPEEK and APEEK were prepared and used as PEMs. All the composite membranes displayed reduced water uptake and swelling ratio compared with pristine SPEEK. The mechanical stability of membranes was greatly improved due to the intermolecular ionic cross-linking between the amino groups of APEEK and the sulfonic acid groups of SPEEK. The crucial properties of PEMs such as proton conductivity and methanol permeability were also investigated. All the composite membranes displayed slightly reduced proton conductivity, improved dimensional stability and greatly reduced methanol permeability. The relative selectivity results indicated that the SPEEK/APEEK composite membranes are very promising for PEM applications.Acknowledgements
The authors gratefully acknowledge the financial support of this work by the Natural Science Foundation of China (21474036 and 21374034) and Science and the Fok Ying-Tong Education Foundation for Young Teachers in the Higher Education Institutions of China (142010).Notes and references
- V. Neburchilov, J. Martin, H. J. Wang and J. J. Zhang, J. Power Sources, 2007, 169, 221–238 CrossRef CAS.
- B. J. Liu, W. Hu, G. P. Robertsona and M. D. Guiver, J. Mater. Chem., 2008, 18, 4675–4682 RSC.
- T. Suda, K. Yamazaki and H. Kawakami, J. Power Sources, 2010, 195, 4641–4646 CrossRef CAS.
- M. A. Hickner, H. Ghassemi, Y. S. Kim, B. R. Einsla and J. E. McGrath, Chem. Rev., 2004, 104, 4587–4612 CrossRef CAS PubMed.
- B. Yameen, A. Kaltbeitzel, A. Langner, H. Duran, F. Muller, U. Gosele, O. Azzaroni and W. Knoll, J. Am. Chem. Soc., 2008, 130, 13140–13144 CrossRef CAS PubMed.
- O. Savadogo, J. New Mater. Electrochem. Syst., 1998, 1, 47–66 CAS.
- T. Higashihara, K. Matsumoto and M. Ueda, Polymer, 2009, 50, 5341–5357 CrossRef CAS.
- C. H. Park, C. H. Lee, M. D. Guiver and Y. M. Lee, Prog. Polym. Sci., 2011, 36, 1443–1498 CrossRef CAS.
- Y. B. Di, W. J. Yang, X. J. Li, Z. Zhao, M. R. Wang and J. M. Dai, RSC Adv., 2014, 4, 52001–52007 RSC.
- Y. H. Yin, J. H. Wang, S. T. Jiang, X. Yang, X. Y. Zhang, Y. Cao, L. Cao and H. Wu, RSC Adv., 2015, 5, 75434–75441 RSC.
- B. J. Liu, G. P. Robertson, D. S. Kim, M. D. Guiver, W. Hu and Z. H. Jiang, Macromolecules, 2007, 40, 1934–1944 CrossRef CAS.
- Y. Chikashige, Y. Chikyu, K. Miyatake and M. Watanabe, Macromolecules, 2005, 38, 7121–7126 CrossRef CAS.
- F. Wang, M. Hickner, Y. S. Kim, T. A. Zawodzinski and J. E. McGrath, J. Membr. Sci., 2002, 197, 231–242 CrossRef CAS.
- S. G. Feng, Y. M. Shang, S. B. Wang, X. F. Xie, Y. Z. Wang, Y. W. Wang and J. M. Xu, J. Membr. Sci., 2010, 346, 105–112 CrossRef CAS.
- J. Jouanneau, R. Mercier, L. Gomom and G. Gebel, Macromolecules, 2007, 40, 9435–9442 CrossRef.
- C. H. Shen, L. C. Jheng, S. L. C. Hsu and J. T. W. Wang, J. Mater. Chem., 2011, 21, 15660–15665 RSC.
- H. Beydaghi, M. Javanbakht, A. Bagheri, P. Salarizadeh, H. G. Zahmatkesh, S. Kashefic and E. Kowsaria, RSC Adv., 2015, 5, 74054–74064 RSC.
- C. X. Zhao, D. He, Y. T. Li, J. F. Xiang, P. Li and H. J. Sue, RSC Adv., 2015, 5, 47284–47293 RSC.
- T. Y. Na, K. Shao, J. Zhu, H. C. Sun, D. Xu, Z. Q. Zhang, C. M. Lew and G. Zhang, J. Power Sources, 2013, 230, 290–297 CrossRef CAS.
- R. K. Nagarale, G. S. Gohil and V. K. Shahi, J. Membr. Sci., 2006, 280, 389–396 CrossRef CAS.
- L. S. Wang, A. N. Lai, C. X. Lin, Q. G. Zhang, A. M. Zhu and Q. L. Liu, J. Membr. Sci., 2015, 492, 58–66 CrossRef.
- Y. Y. Sun, X. M. Wu, D. X. Zhen, S. K. Zhang, M. M. Hu and G. H. He, J. Appl. Polym. Sci., 2016, 133, 42867 Search PubMed.
- H. T. Pu, Y. J. Qin, L. M. Tang, X. R. Teng and Z. H. Chang, Electrochim. Acta, 2009, 54, 2603–2609 CrossRef CAS.
- A. Muthumeenal, S. Neelakandan, P. Kanagaraj and A. Nagendran, J. Renewable Energy, 2016, 86, 922–929 CrossRef CAS.
- M. Erkartal, H. Usta, M. Citir and U. Sen, J. Membr. Sci., 2016, 499, 156–163 CrossRef CAS.
- H. T. Li, G. Zhang, W. J. Ma, C. J. Zhao, Y. Zhang, M. M. Han, J. Zhu, Z. G. Liu, J. Wu and H. Na, Int. J. Hydrogen Energy, 2010, 35, 11172–11179 CrossRef CAS.
- H. Q. Zhang, X. F. Li, C. J. Zhao, T. Z. Fu, Y. H. Shi and H. Na, J. Membr. Sci., 2008, 308, 66–74 CrossRef CAS.
- X. F. Li, C. P. Liu, H. Lu, C. J. Zhao, Z. Wang, W. Xing and H. Na, J. Membr. Sci., 2005, 255, 149–155 CrossRef CAS.
- W. M. Li, Z. M. Cui, X. C. Zhou, S. B. Zhang, L. Dai and W. Xing, J. Membr. Sci., 2008, 315, 172–179 CrossRef CAS.
- M. M. Guo, B. J. Liu, Z. Liu, L. F. Wang and J. Z. H. Jiang, J. Power Sources, 2009, 189, 894–901 CrossRef CAS.
- R. Perrin, M. Elomaa and P. Jannasch, Macromolecules, 2009, 42, 5146–5154 CrossRef CAS.
- N. W. Li, Z. M. Cui, S. B. Zhang and W. Xing, Polymer, 2007, 48, 7255–7263 CrossRef CAS.
- T. Miyahara, T. Hayano, S. Matsuno, M. Watanabe and K. Miyatake, ACS Appl. Mater. Interfaces, 2012, 4, 2881–2884 CAS.
| This journal is © The Royal Society of Chemistry 2016 |