Highly efficient and selective hydrogenation of chloronitrobenzenes to chloroanilines by H2 over confined silver nanoparticles†
Lini
Yang
a,
Lina
Xing
a,
Cunxia
Cheng
a,
Lixin
Xia
*a and
Hongyang
Liu
*b
aDepartment of Chemistry, Liaoning University, Shenyang, Liaoning 110036, China 110036. E-mail: lixinxia@lnu.edu.cn; Fax: +86 2462202380; Tel: +86 2462202380
bShenyang National Laboratory for Materials Science, Institute of Metal Research, Chinese Academy of Sciences, Shenyang, Liaoning 110016, China. E-mail: liuhy@imr.ac.cn; Fax: +86 2483970019; Tel: +86 2423971577
First published on 24th March 2016
Abstract
Ag NPs with sizes ranging form 5–6 nm confined into mesoporous silica SBA-15 (Ag-NPs/SBA-15) are successfully prepared by a facile and rapid glucose/glycol assisted one-step vacuum impregnation method. The as-prepared Ag-NPs/SBA-15 catalyst shows very good activity and selectivity for hydrogenation of chloronitrobenzenes to the corresponding chloroanilines by H2 compared with that of the SiO2 supported Ag catalysts. The robust catalytic performance of the as-prepared Ag-NPs/SBA-15 catalyst displays its potential application for the production of chloroanilines in the fine chemicals industry.
Introduction
Chloroanilines (CANs) are important and valuable industrial intermediates for a variety of agrochemicals, pharmaceuticals, dyes and pigments.1 Currently, the commercial production of CANs mainly uses non-catalytic reduction of the corresponding chloronitrobenzenes (CNB) with stoichiometric reducing agents such as sodium hydrosulfite, iron, tin, or zinc in aqueous ammonia.2 However these processes are not environmentally sustainable. Therefore, it is desirable to produce these intermediates by selective reduction of the corresponding CANs with H2 using supported metal catalysts. A number of noble metal catalysts based on Pt, Pd, Ru, Rh, Ir, Au, Ag etc. were reported to be efficient for the hydrogenation of this reaction.3–14 Among these noble metals, even though Ag is less active than Pt-group catalysts, lower catalyst costs still render its use attractive. Besides that, the hydrodechlorination of CAN to the byproduct aniline (AN) can be fully suppressed at complete conversion of CNB over the Ag catalysts.15Generally, for the supported noble metal catalysts, the progressive deactivation of catalysts is a major economic concern and mastering their stability has become as essential as controlling their activity and selectivity. Thus, there is a strong motivation to develop facile and feasible approaches for preparing the stable and recyclable supported Ag catalysts for the hydrogenation of CNBs to the corresponding CANs by H2. Recently, mesoporous silica has been considered as a good catalytic support, which is attributed to its high BET surface areas, uniform and tunable pore sizes. Metal NPs confined into mesoporous silica such as MCM-41, SBA-15, etc. have been widely investigated and showed robust catalytic performance in heterogeneous catalytic reactions.16 In this study, we report a facile glucose/glycol assisted one-step vacuum impregnation method to rapidly fabricate Ag NPs confined into mesoporous SBA-15 matrix. The mesoporous SBA-15 is employed as the template, glycol works as the reducing agent and glucose is used as the protecting agent. In the whole preparing process, no organosilane groups are used, and no hydrogen treatment were needed to obtain metallic Ag NPs confined in he SBA-15 matrix.17 The obtained Ag NPs are uniformly assembled in the channels of mesoporous SBA-15. The as-prepared Ag-NPs/SBA-15 catalyst shows a very good activity and selectivity for hydrogenation of CNBs to the corresponding CANs, comparing with the traditional SiO2 supported Ag catalyst (Ag/SiO2). The robust recycle ability of the as-prepared Ag-NPs/SBA-15 catalyst displays its great potential as an available and cheap noble catalyst for the production of CANs by the hydrogenation reaction in the fine chemical production.
Experimental section
Synthesis of SBA-15
The mesoporous silica SBA-15 was synthesized using a literature approach.18 Briefly, a solution of P123![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCl
HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TEOS
TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 2
H2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.6
3.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 (mass ratio) was prepared, stirred for 24 h at 40 °C, and then heated at 100 °C for 2 days. The final products were washed, dried and calcined at 550 °C for 6 h.
60 (mass ratio) was prepared, stirred for 24 h at 40 °C, and then heated at 100 °C for 2 days. The final products were washed, dried and calcined at 550 °C for 6 h.
Synthesis of SBA-15 confined Ag nanoparticles and nanorods
1 g of parent SBA-15 was dehydrated at 110 °C vacuum for 4 h. Then, a mixture of 2 ml aqueous solution that contains 1 ml glycol, 100 mg glucose, 1 ml water, 0.5 ml NH3·H2O and 63 mg AgNO3 was infiltrated in SBA-15 by incipient wetness impregnation at room temperature. The impregnated samples was quickly transported into the oven and allowed to react at 100 °C for 6 h. After that, the products were then washed, dried, and collected. Finally, Ag nanoparticles were found to be embedded in the SBA-15 host with 4 wt%. The Ag nanorods were synthesized by the similar process, but using just glycol as the reducing reagent.Synthesis of SiO2 sphere supported Ag nanoparticles
Mono-dispersed silica nanoparticles were prepared by Stöber method.19 After that, 2 ml (3-aminopropyl)triethanoxysilane (APTES) was added under vigorously stirring to get 2 g APTES-functionalized silica nanoparticles (NH2–SiO2). 2.0 g of the NH2–SiO2 was added to 30 ml of an aqueous solution of HCHO (37%), which was stirred at 60 °C for 2 h, filtered and dried in air at 120 °C for 3 h, finally a white solid powder (denoted as CH2O–SiO2) was made. 1.0 g of CH2O–SiO2 was suspended in a solution of 63 mg AgNO3 dissolved in 40 ml deionized water, after being stirred at 60 °C for 2 h, and following filtering and drying in air at 120 °C for 12 h, the Ag/SiO2 catalyst was obtained. The loading of silver was found to be 4.0 wt% in weight.Catalytic study and characterization
The catalytic hydrogenation of CNBs was carried out in an autoclave under the following reaction conditions: 140 °C, 0.5–3.0 MPa, 0.1 g of catalyst, 0.5 g of substrates, and 25 ml of ethanol. After completion of the reaction, the reactor was cooled to room temperature and hydrogen gas was discharged. The catalyst was filtered and fresh ethanol was added to the catalyst and refluxed for 2 h. After cooling to room temperature, the catalyst was filtered and dried at 120 °C for 2 h, waiting for reuse. The speed of the agitation was set at 500 rpm in order to inhibit the mass transfer limitation in the hydrogenation reaction. The products were analyzed by gas chromatography (Agilent 7890) equipped with a FID detector. Transmission electron microscopy (TEM) was performed by a Tecnai G2 F20 S-TWIN electron microscope operated at 200 kV. The specific surface area was measured by the Brunauer–Emmett–Teller (BET) method using nitrogen adsorption–desorption isotherms on a Micrometrics ASAP 3020 system. X-ray diffraction (XRD) was conducted on a Philips diffractometer using Cu Kα radiation. The elemental analysis was performed using an inductively-coupled plasma-atomic emission spectrometer (ICP-AES, IRIS Intrepid).Results and discussion
Fig. 1 shows the schematic procedure for the fabrication of the Ag nanostructures confined into the SBA-15 support. The template free SBA-15 (TEM images in Fig. S1†) was dehydrated at 383 K for about 4 h under vacuum. After cooling to room temperature, a mixture of solution that contains 1 ml glycol, 100 mg glucose, 1 ml water, 0.5 ml NH3·H2O and 63 mg AgNO3 was infiltrated in the 1 g SBA-15 by wetness impregnation at room temperature. The impregnated samples was quickly transported into the oven and allowed to react at 100 °C for 6 h. After that, the products were then washed, dried, and collected. Finally, Ag nanoparticles were found to be embedded in the SBA-15 host with 4 wt% confirmed by the ICP analysis. Ag nanorods (NRs) were synthesized by the similar process, but just using glycol as the reducing reagent with the absence of glucose. | ||
| Fig. 1 Schematic illustrating the preparation of Ag nanoparticles and nanorods in the channel of the SBA-15. | ||
TEM images provide direct observation of the morphology and distribution of Ag-NPs in the SBA-15 matrix. Fig. 2a and b displayed the TEM images of the as-prepared Ag-NPs/SBA-15. It can be observed that many black dots corresponding to the Ag-NPs as shown in Fig. 2a are uniformly distributed in the channel of the SBA-15. The typical TEM images of Fig. 2b and the inset of Fig. 2b clearly show that the obtained Ag-NPs are in the size around 5–6 nm, and almost exclusively stayed inside the channels of host SBA-15. Meanwhile, we can see that highly ordered mesoporous structure is still well maintained after incorporation of Ag-NPs, compared with that of the calcined SBA-15. HRTEM image of the Ag-NPs (Fig. 2c) indicates that the obtained Ag-NPs presented the face-centered cubic (FCC) Ag lattice with an Ag (111) interlayer spacing of 0.23 nm. Fig. 2d shows the STEM images of the obtained Ag-NPs/SBA-15 samples. Notably, the Ag nanoparticles are uniformly distributed in the channels of SBA-15 support, consisting well with the TEM images (Fig. 2a and b). The EDX result reveals the coexistence of dominated Ag and Si elements in the obtained nanocomposite. The TEM results confirm that the glucose/glycol assisted vacuum impregnation is a rapid and facile method to obtain fine and highly dispersed Ag-NPs in comparison with the previous reported methods.20–25
The low-angle XRD patterns of the calcined SBA-15 displayed in Fig. S2a† exhibit intense diffraction peaks, characteristic of a two-dimensional hexagonal (P6mm) structure with d100 spacing of ca. 7.0 nm. After the formation of Ag NPs in the channels of SBA-15, the low-angle XRD patterns are found to be well maintained, indicating that the mesoporous structure is still well kept after incorporation of Ag NPs, consistent with the TEM results. Meanwhile, it can be observed that the intensities of the peaks slightly decreased, implying the Ag-NPs have been encapsulated inside the channels of the SBA-15.26
Nitrogen-sorption isotherms for calcined SBA-15 and Ag-NPs/SBA-15 are shown in Fig. S2b.† Table S1† summarizes the results of N2-sorption analyses. The isotherms feature hysteresis loops with sharp adsorption and desorption branches in the P/P0 range of 0.5–0.8, featuring typical of mesoporous structure solids. After the incorporation of Ag-NPs, the BJH surface (SBJH) and total pore volume (Vt) decrease from 680 to 570 m2 g−1 and from 0.93 to 0.75 cm3 g−1, respectively, with a slight decrease of the average pore size (DBJH) from 6.8 to 5.9 nm. This suggests that most of the nanometer-scaled void space of the host silica is still open, which is important for molecules to diffuse into the host silica, and to contact with Ag-NPs for catalytic reactions.27 The wide angle XRD pattern of Ag-NPs/SBA-15 (Fig. S2c†) presents two distinct peaks contributed by Ag metal. These peaks are very broad and weak, indicating the nanocrystalline nature of Ag in the nanocomposite. From the peak width of Ag (111) reflection by using Scherrer's equation with a spherical model for approximation, the average Ag particle size was estimated to be 5.6 nm consisting well with the TEM results.
The formation of Ag NPs in the channels of SBA-15 with the assistance of glycol and glucose is attributed to two important factors. Firstly, SBA-15 template can act as a limiting factor to restrain the growth of Ag in one dimension under the reduction of ethylene glycol. Another important factor is that the glucose can prevent the migration of as-prepared Ag NPs to form the Ag NRs. We find that the Ag NRs as presented in Fig. S3† can be facilely fabricated, when we use just glycol as the reducing agent with the absence of glucose.
The hydrogenation of o-chloronitrobenzene (o-CNB) was employed as a probe reaction to test the activity and selectivity of the as-prepared Ag-NPs/SBA-15 catalysts. A SiO2 sphere supported Ag NPs with sizes range from 5–6 nm as the controlled catalyst was also prepared by a modified method.5 The TEM and STEM images of the controlled Ag/SiO2 catalyst were shown in Fig. 3. The catalytic performance of the Ag-NPs/SBA-15 and Ag/SiO2 catalysts are summarized in Table 1. At the initial reaction pressure of 0.5 MPa, the conversion of o-CNB over the Ag-NPs/SBA-15 catalyst was only 23.1% (entry 1). Increasing the reaction pressure to 2.0 MPa, the o-CNB conversion increased to 100% dramatically (entry 2), the selectivity to o-chloroaniline (o-CAN) reached 99.2%, indicating that Ag-NPs/SBA-15 is a good catalyst for selective hydrogenation of o-CNB to o-CAN. Further increasing the reaction pressure to 3.0 MPa, no obvious changes in conversion or selectivity were noted (entry 3). And a 98.2% o-CBN conversion and a 99.0% o-CAN selectivity were obtained over the Ag/SiO2 catalyst (entry 4). The catalytic reduction of various CNBs over Ag-NPs/SBA-15 was also examined. In all the cases, the selectivity to CANs was >99% with 100% CNB conversion (Table S2†). The TOF of o-CNB over the as-prepared Ag-NPs/SBA-15 (entry 2) was 41.7 h−1, which is lower than that of the supported Pt catalysts,6b but it is comparable with some of the reported Au or Ir catalysts.6c,13b
 | ||
| Fig. 3 (a and b) TEM images of the controlled Ag/SiO2, (c) STEM image of the obtained Ag/SiO2, (d) HRTEM image of one single Ag NP supported on the SiO2. | ||
| Entry | Catalyst | Time (h) | P (Ma) | Con (%) | Sel (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 0.1 g of the catalyst, 4 wt% Ag, 0.5 g of the substrate and 25 ml of ethanol, T = 140 °C. | |||||
| 1 | Ag-NPs/SBA-15 | 3 | 0.5 | 23.1 | 99.2 |
| 2 | Ag-NPs/SBA-15 | 3 | 2 | 100 | 99.5 |
| 3 | Ag-NPs/SBA-15 | 3 | 3 | 100 | 99.1 |
| 4 | Ag-NPs/SiO2 | 3 | 2 | 98.2 | 99.0 |
| 5 | Ag-NPs/SBA-15 | 2.5 | 2 | 85.6 | 99.3 |
| 6 | Ag-NPs/SiO2 | 2.5 | 2 | 82.3 | 99.1 |
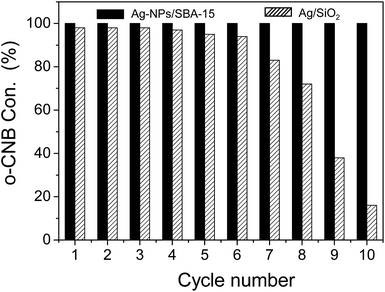
The stability of Ag-NPs/SBA-15 catalyst was also investigated by repeated measurements under the similar conditions (entry 3 and entry 4). The catalytic results were displayed in Fig. 4 and S4.† After each run, the catalyst was recycled by centrifugation, washing and drying for the next run. It can be seen that the Ag-NPs/SBA-15 catalyst exhibits robust catalytic stability with a 100% o-CNB conversion and a 99.0% o-CAN selectivity even after 10 cycle hydrogenation reaction. However, when the Ag@SiO2 was used, after 10 runs, only a 16.3% o-CNB conversion and an 85.2% o-CAN selectivity was obtained, comparing with the initial catalytic performance with a 98.2% o-CNB conversion and a 99.0% o-CAN selectivity, which indicates that the as-prepared Ag-NPs/SBA-15 catalyst has higher catalytic recycle ability than that of the Ag/SiO2 catalyst. Notably, when the reaction time was reduced from 3 h to 2.5 h, an 85.6% and 82.3% o-CNB conversion were respectively obtained over the Ag-NPs/SBA-15 and Ag/SiO2 catalysts as displayed in the Table 1 (entry 5 and entry 6). Under this kinetic control condition, the catalytic stability of hydrogenation of o-CNB to o-CAN over the Ag-NPs/SBA-15 and Ag/SiO2 catalysts were tested, it was still found that the Ag-NPs/SBA-15 displayed robust catalytic stability in the hydrogenation reaction, comparing with that of the Ag/SiO2 catalyst as shown in Fig. S5.†
In general, for the liquid catalytic hydrogenation reaction, the stirring and washing process lead to the lost of supported metal nanoparticles and the deactivation of catalysts. In order to investigate the distinct promotion of catalytic stability over the as-prepared Ag-NPs/SBA-15 catalyst, the TEM images of the used Ag-NPs/SBA-15 and Ag/SiO2 catalysts after 10 runs are displayed in Fig. 5. Note that the density of the Ag NPs in the used Ag-NPs/SBA-15 catalysts (TEM images in Fig. 5a and b) almost did not change, comparing with that of the fresh Ag-NPs/SBA-15 catalysts (TEM images in Fig. 2a and b), and the weight loading of the Ag NPs was 3.9 wt% confirmed by the ICP analysis, revealing that the confinement effect will significantly prevent the lost of Ag nanoparticles during the hydrogenation reaction and promote the stability of supported Ag catalysts. However, as to the controlled Ag/SiO2 catalysts, the weight loading of Ag was just 0.85 wt% after 10 runs, and the density of the Ag NPs in the used Ag/SiO2 catalysts was much lower than that of the fresh Ag/SiO2 catalyst (TEM images in Fig. 5c and d), probably resulting from the lost of Ag nanoparticles supported on the SiO2 surface during the recycling reaction. Apparently, the presence of confined structure are sufficient to stabilize the Ag NPs by inhibiting their leaching or aggregation during the catalytic reaction, resulting in the excellent recycle ability over the Ag-NPs/SBA-15 catalyst.
 | ||
| Fig. 5 TEM images of the (a and b) Ag-NPs/SBA-15 and (c and d) Ag/SiO2 catalysts after using 10 runs. | ||
Conclusions
In summary, Ag NPs confined into SBA-15 are successfully prepared through a facile and feasible glucose/glycol assisted one-step vacuum impregnation method. The as-prepared Ag-NPs/SBA-15 catalyst shows a very good activity and selectivity for hydrogenation of CNBs to the corresponding CANs. A 100% o-CNB conversion and a 99% o-CAN selectivity were obtained over the Ag-NPs/SBA-15 catalyst even after 10 cycles of the hydrogenation reaction. The robust recycle ability over the Ag-NPs/SBA-15 catalyst displays its potential application as an effective catalyst for the selective hydrogenation of various chloronitrobenzenes by H2, comparing with that of the traditional SiO2 supported Ag catalysts.Acknowledgements
This work was supported by NSFC (21203083, 21271095, 21573254, 91545110), the Education Bureau of Liaoning Province (L20111003). The Institute of Metal Research and Youth Innovation Promotion Association (CAS) of the Chinese Academy of Sciences.Notes and references
- V. Kratky, M. Kralik and M. Hronec, Appl. Catal., A, 2002, 235, 225 CrossRef CAS.
- A. Corma and P. Serna, Science, 2006, 313, 332 CrossRef CAS PubMed.
- M. Pietrowski, Green Chem., 2011, 13, 1633 RSC.
- C. Lian, H. Q. Liu, C. Xiao, Y. Liu and Y. Wang, Chem. Commun., 2012, 48, 3124 RSC.
- Y. Y. Chen, C. Wang, H. Y. Liu, J. S. Qiu and X. H. Bao, Chem. Commun., 2005, 5298 RSC.
- (a) L. Liu, B. Qiao, Z. Chen, J. Zhang and Y. Deng, Chem. Commun., 2009, 653 RSC; (b) C. Lian, H. Liu, C. Xiao, W. Yang and Y. Wang, Chem. Commun., 2012, 48, 3124 RSC; (c) D. P. He, H. Shi, Y. Wu and B. Q. Xu, Green Chem., 2007, 9, 849 RSC.
- F. Wang, J. Liu and X. Xu, Chem. Commun., 2008, 2040 RSC.
- A. Corma, P. Serna, P. Conception and J. J. Calvino, J. Am. Chem. Soc., 2008, 130, 8748 CrossRef CAS PubMed.
- D. He, H. Shi, Y. Wu and B. Q. Xu, Green Chem., 2007, 9, 849 RSC.
- K. I. Shimizu, Y. Miyamoto and A. Satsuma, J. Catal., 2010, 270, 86 CrossRef CAS.
- Z. M. Rong, W. Q. Du, Y. Wang and L. H. Lu, Chem. Commun., 2010, 46, 1559 RSC.
- Y. Hu, K. Tao, C. Wu, C. Zhou, H. Yin and S. Zhou, J. Phys. Chem. C, 2013, 117, 8974 CAS.
- (a) F. A. Harraz, H. M. Killa and I. A. Ibrahim, J. Catal., 2012, 286, 184 CrossRef CAS; (b) T. Lu, H. Wei, X. Yang, X. D. Wan and T. Zhang, Langmuir, 2015, 31, 90 CrossRef CAS PubMed; (c) M. Pietrowski, Green Chem., 2011, 13, 1633 RSC.
- (a) H. Q. Lin, J. W. Zheng, Y. Z. Yuan and Y. H. Yang, J. Catal., 2015, 184, 330 Search PubMed; (b) X. K. Wang, Q. Liu, Z. Xiao and C. Liang, RSC Adv., 2014, 4, 48254 RSC.
- H. Blaser, H. Steiner and M. Studer, ChemCatChem, 2009, 1, 210 CrossRef CAS.
- J. M. Sun, D. Ma and X. H. Bao, J. Am. Chem. Soc., 2006, 128, 15756 CrossRef CAS PubMed.
- (a) M. H. Huang, A. Choudrey and P. D. Yang, Chem. Commun., 2000, 12, 1063 RSC; (b) J. Li, C. Tsai and S. Kuo, RSC Adv., 2015, 5, 42798 RSC.
- D. Y. Zhao and G. D. Stucky, Science, 1998, 279, 548 CrossRef CAS PubMed.
- W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62 CrossRef.
- P. Verma, Y. Kuwahara, K. Mori and H. Yamashita, J. Mater. Chem. A., 2015, 3, 18889 CAS.
- (a) X. Y. Liu, A. Q. Wang, X. D. Wang, C. Y. Mou and T. Zhang, Chem. Commun., 2008, 27, 3187 RSC; (b) Q. Geng and J. Du, RSC Adv., 2014, 4, 16425 RSC.
- (a) H. Liu, D. Ma and X. H. Bao, Chem. Commun., 2008, 12, 1063 Search PubMed; (b) S. Sareen, V. Mutreja, S. Singh and B. Pal, RSC Adv., 2015, 5, 184 RSC; (c) H. Liu, D. Ma and X. H. Bao, Dalton Trans., 2009, 1894 RSC.
- P. Saint-Cricq, J. Wang and T. Okubo, J. Mater. Chem. B, 2013, 1, 2451 RSC.
- J. Han, P. Fang, W. Jiang, L. Li and R. Guo, Langmuir, 2012, 28, 4768 CrossRef CAS PubMed.
- Y. Xie, B. Yan, C. Tian, Y. Liu, Q. Liu and H. Zeng, J. Mater. Chem. A, 2014, 2, 17730 CAS.
- (a) L. Yang and M. S. Jin, CrystEngComm, 2013, 15, 2804 RSC; (b) L. Yang, X. Song, L. X. Xia and M. S. Jin, J. Mater. Chem. A, 2013, 1, 7316 RSC.
- P. H. Liu, Y. F. Ho and C. M. Yang, Chem. Mater., 2003, 15, 275 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: XRD, BET, TEM results. See DOI: 10.1039/c6ra02288j |
| This journal is © The Royal Society of Chemistry 2016 |