Preparation of thiol-functionalized magnetic sawdust composites as an adsorbent to remove heavy metal ions†
Wentao Gana,
Likun Gaoa,
Xianxu Zhanb and
Jian Li*a
aKey Laboratory of Bio-based Material Science and Technology, Ministry of Education, Material Science and Engineering College, Northeast Forestry University, No. 26 Hexing Road, Xiangfang District, Harbin 150040, China. E-mail: jlnefu@163.com; Tel: +86 0451 82192399
bDehua TB New Decoration Material Co., Ltd, Huzhou, 313200, P.R. China
First published on 11th April 2016
Abstract
Thiol-functionalized magnetic sawdust, synthesized by precipitating γ-Fe2O3 nanoparticles on the sawdust surface and then modifying the 3-mercaptopropyltrimethoxysilane layers, has been investigated as an environmentally friendly and recyclable adsorbent for heavy metal ions. The process of modifying was confirmed by scanning electron microscopy, Fourier transform infrared spectroscopy, X-ray photoelectron spectroscopy and X-ray diffraction. Compared with the nonmagnetic sawdust, the thiol-functionalized magnetic sawdust possesses high saturation magnetization (7.28 emu g−1), and can be easier and faster to separate from water under an external magnetic field. The adsorption equilibrium was reached within 20 min and the adsorption kinetics were elucidated by a pseudo-second-order model. The adsorption isotherms of Cu2+, Pb2+ and Cd2+ fitted well with the Langmuir model, exhibiting an adsorption capacity of 5.49 mg g−1, 12.5 mg g−1 and 3.80 mg g−1, respectively. Competitive adsorption among the three metal ions showed a preferential adsorption of Pb2+ > Cu2+ > Cd2+. In addition, the magnetic sawdust adsorbent exhibited excellent acid–alkali stability and the metal-loaded adsorbent was able to regenerate in an acid solution without significant adsorption capacity loss.
1. Introduction
Heavy metal ions such as cadmium, lead and copper, often found in industrial wastewater, are non-biodegradable materials in the environment and can be accumulated in human bodies throughout the food chain, causing significant physiological disorders.1–3 Therefore, it is necessary to remove heavy metal ions from wastewater before discharge. A variety of methods and techniques have been developed for the removal of toxic heavy metal ions from aqueous solutions, such as chemical precipitation, adsorption, ion exchange, and membrane separation, etc.4–6 Considering the efficiency and operation of the available methods, adsorption is regarded as one of the best available control techniques for the removal of low concentrations of heavy metals from wastewater. However, some effective adsorbents such as activated carbon and ion exchange resins are not suitable for practical applications owing to the high capital costs.The application of low cost adsorbents has been investigated as a replacement for costly conventional technologies of removing heavy metal ions from solution. Plant wastes have increasingly received more attention because they are abundant in nature, biodegradable, environmental friendly, and renewable.7 A lot of adsorption experiments have been concentrated on plant wastes such as waste tea,8 orange peel,9 chestnut shell,10 sugarcane bagasse,11 Cinnamomum camphora leaves,12 neem bark,13 sawdust,14 etc. These plant wastes are mostly composed of cellulose, hemicellulose and lignin, both with a capacity for binding metal cations. However, the adsorption capacity of these untreated plant wastes is usually limited by the low quantity of functional groups. The direct use of the untreated materials as absorbents not only can increases chemical oxygen demand (COD) of water due to the release of soluble organic compounds such as lignin, pectin and tannin into the solution,15 but also requires an additional separation step to remove such absorbents from solution. Therefore, the design and exploration of recyclable biosorbents based on plant wastes are still necessary.
Some studies have proved that the chemical modification could be used to enhance the adsorption capacity of plant waste through introducing functional groups and decrease the COD of water by the release of the organic compounds in the chemical pretreatment process.16 Functionalized silica layer with organic functional groups are commonly used since the silica provides many advantages such as good adsorption, cation exchange capacity, easy to impregnate medium to create sever modified silica surface with high mechanic strength and thermal stability.17 Among the types examined, those with thiol-functionalised groups have been found to be efficient for the removal of heavy metal ions.18 Moreover, Merk et al. has confirmed that the wood materials can act as efficient substrates to the nucleation and growth of magnetic particles.19 Many researches also have proved that the wood/magnetic particles composites with appropriate magnetic property could be collected by the permanent magnet.20,21 As an abundant by-product, sawdust with the porous structure and a hydrophilic nature, obtained from mechanical wood processing, which is a renewable and low cost waste materials, is an excellent scaffold for further magnetic particles modification. Furthermore, wood sawdust is mainly composed of cellulose (45–50%) and lignin (23–30%), both has a capacity for binding metal cations because of the hydroxyl, phenolic and carboxylic groups present in their structure.22
In this study, poplar sawdust was chemically modified by magnetic γ-Fe2O3 nanoparticles and 3-mercaptopropytrimethoxysilane (MPTMS), used as a kind of recyclable adsorbent to remove heavy metal ions from water. Thiol-functionalized magnetic sawdust (TF-MS) were characterized using scanning electron microscope (SEM), X-ray diffraction (XRD), X-ray photoelectron spectroscope (XPS), Fourier transform infrared spectroscopy (FTIR), thermogravimetric analyses (TGA) and magnetization measurement. Also the adsorption behaviors including kinetic, isotherm, competitive adsorption and stability of TF-MS were evaluated.
2. Materials and methods
2.1. Materials
Poplar sawdust used for this experiment was obtained from a local saw mill in Heilongjiang Province, China. The sawdust was first washed with distilled water, dried, cut, and sieved through 100-mesh screen. The ferric chloride hexahydrate (FeCl3·6H2O), ferrous chloride tetrahydrate (FeCl2·4H2O), ammonia solution (25%), anhydrous ethanol (≥99.9%), 3-mercaptopropyltrimethoxysilane (MPTMS, ≥95%), glacial acetic acid (≥99.5%) used in this study were supplied by Shanghai Boyle Chemical Company Limited., and used without further purification.2.2. Synthesis of magnetic sawdust composites (MS)
TF-MS was prepared as shown in Fig. 1. Firstly, 10 g wood sawdust, 5.40 g FeCl3·6H2O (0.02 mol) and 1.98 g FeCl2·4H2O (0.01 mol) were dissolved in 200 mL distilled water, the Fe3+/Fe2+ ratio was 2. The pH value of the iron ions precursors were adjusted to ca. 10 by the dropwise addition of 10% ammonia solution. After stirring for 15 min, the system was transferred into a Teflon-lined stainless-steel autoclave, and heated at 90 °C to precipitate the magnetic Fe3O4 on the sawdust substrate. Then the oxidation reaction was appeared when the Fe3O4 nanoparticles exposure to air, leading to form γ-Fe2O3. Finally, the prepared specimens were removed, rinsed with distilled water for three times, and dried at 50 °C for over 24 h in vacuum.2.3. Surface modification of MS
The 3-mercaptopropyltrimethoxysilane ethanol solution was prepared by stirring the mixture of the 100 mL anhydrous ethanol, 5 mL 3-mercaptopropyltrimethoxysilane (MPTMS), 2 mL H2O, 5 mL glacial acetic acid at room temperature. 10 g magnetic sawdust was immersed into the 3-mercaptopropyltrimethoxysilane ethanol solution. The modification was maintained at 60 °C for 12 h. In a simplified scheme (Fig. 1), the silanization reaction was described as following: firstly, the organosilane was placed into an aqueous solution of an acid that acts as a catalyst. It is hydrolyzed, the methoxy groups (–OCH3) are replaced by hydroxyl groups to form reactive silanol groups. Then, the hydrolyzed silane condensed with the hydroxyl groups on the surface of magnetic sawdust forming a covalent bond with OH groups. With the continuous dehydration reaction, the –SH groups were successfully formed onto the surface of magnetic sawdust. The reaction product was collected with a magnet, washed repeatedly with ethanol and distilled water, dried in a vacuum oven at 50 °C.2.4. Characterization
The morphology of the samples was examined by the scanning electron microscopy (SEM, FEI, and Quanta 200). The crystalline structure of these composites were identified using the X-ray diffraction (XRD, Rigaku, and D/MAX 2200) operating with Cu Kα radiation (λ = 1.5418 Å) at a scan rate (2θ) of 4° min−1, an accelerating voltage of 40 kV, and an applied current of 30 mA ranging from 5° to 80°. The X-ray photoelectron spectroscope (XPS) was recorded on the Thermo ESCALAB 250XI. The deconvolution of the overlapping peaks was performed using a mixed Gaussian–Lorentzian fit program. The surface chemical compositions of the samples were determined via the Fourier transformation infrared spectroscopy (FTIR, Nicolet, Magna-IR 560). Thermogravimetric analyses (TGA) were examined using a thermal analyzer (TGA, SDT Q600) in the temperature range from room temperature up to 800 °C at a heating rate of 10 °C min−1, with a flow of dried air (100 mL min−1). The magnetization measurements were carried out at room temperature using a superconducting quantum interference device (MPMS XL-7, Quantum Design Corp.). The potassium dichromate method was used to determine the chemical oxygen demand (COD).2.5. Batch adsorption experiments
Batch experiments were conducted in duplicates in 250 mL glass conical flasks under ultrasonication for several minutes at 25 °C. An amount of 0.1 g adsorbent and 100 mL heavy metal solution was used for every treatment unless otherwise stated. Separation of the adsorbent was completed in 1 min by applying an external magnet. The concentration of heavy metal was determined by the Flame Atomic Absorption Spectrometer (TAS-990AFG). The equilibrium sorption capacity, qe, was calculated according to eqn (1):
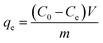 | (1) |
To survey competitive adsorption among Cu2+, Pb2+ and Cd2+, 0.1 g adsorbent was added into 100 mL multi-metal solution with the initial pH 6. For bi-metal solution, the concentration was 5 mg L−1 for each and for tri-metal solution, 3.33 mg L−1 for each metal.
All the experiments were replicated three times, and the mean values were used in our analyses. If the standard error (S.E.) were greater than 0.05, the test was repeated to control for errors.
2.6. Stability and regeneration of adsorbent
Stability of TF-MS under acidic and alkaline conditions was evaluated by dispersing 0.1 g adsorbent in 100 mL different concentration of HCl or NaOH solution. After shaking for 6 h at 25 °C, and then the treated adsorbent was washed to neutrality for reuse. The adsorption by the acid or alkali treated TF-MS was investigated in aqueous solution containing Pb2+ ions (15 mg L−1) at 25 °C for 30 min. By determining the magnetic property and adsorption ability of the treated adsorbent, stability could be inferred.Regeneration studied was conducted by dispersing 0.1 g Pb2+ loaded adsorbent in 100 mL of 1 M HCl solution for 30 min. Then the adsorbent was collected and washed for reused.
3. Results and discussion
3.1. Characterization of adsorbent
Fig. 2 shows the representative SEM images of the untreated sawdust and TF-MS. Fig. 2a shows the typical SEM image of the poplar sawdust surface, the smooth surface and some pits (namely the smallest holes of all pores) found on the walls of tracheids. After chemical treatment, the SEM images of TF-MS (Fig. 2b and c) reveal that many tiny γ-Fe2O3 nanoparticles are precipitated on the surface of the sawdust. There is no detected bare surface area on the surface of sawdust, indicating the high efficiency of our synthesis method.The FTIR spectra of untreated sawdust, MS and TF-MS are shown in Fig. 3. In both spectra, the broad and intense absorption peak at 3420 cm−1 attributed to the O–H stretching vibrations of cellulose, hemicellulose, lignin and absorbed water. The peak observed at 2901 cm−1 corresponded to the C–H stretching vibrations of methyl, methylene and methoxy groups. The peak at 1739 cm−1 in the untreated sawdust spectrum shows the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching vibration of the carboxyl groups of hemicelluloses and lignin in the sawdust. The peak around 1594 cm−1 is due to the aromatic stretching vibration of lignin. The peaks around 1424 cm−1 could be attributed to the C–H deformation vibrations of methyl, methylene and methoxy groups. The peaks in the range 1300–1000 cm−1 could be assigned to the C–O stretching vibration of hemicelluloses and lignin.23 For magnetic sawdust, the peaks at 560 cm−1 and 634 cm−1 attributed to the Fe–O bond vibration of γ-Fe2O3.24 The FTIR spectrum of TF-MS shows the strong adsorption characteristics of Fe–O–Si bands at 587 cm−1.25 There is also presented the characteristic bands of Si–O–Si bonds (νas Si–O–Si at 1106 cm−1, νas Si–OH at 1037 cm−1). S–H stretches were found at 2509 cm−1, which are typically very weak and cannot be detected in the spectra of thin film.26 The results illustrated that the sawdust was functionalized with magnetic particles and 3-mercaptopropyltrimethoxysilane in the synthetic process.
O) stretching vibration of the carboxyl groups of hemicelluloses and lignin in the sawdust. The peak around 1594 cm−1 is due to the aromatic stretching vibration of lignin. The peaks around 1424 cm−1 could be attributed to the C–H deformation vibrations of methyl, methylene and methoxy groups. The peaks in the range 1300–1000 cm−1 could be assigned to the C–O stretching vibration of hemicelluloses and lignin.23 For magnetic sawdust, the peaks at 560 cm−1 and 634 cm−1 attributed to the Fe–O bond vibration of γ-Fe2O3.24 The FTIR spectrum of TF-MS shows the strong adsorption characteristics of Fe–O–Si bands at 587 cm−1.25 There is also presented the characteristic bands of Si–O–Si bonds (νas Si–O–Si at 1106 cm−1, νas Si–OH at 1037 cm−1). S–H stretches were found at 2509 cm−1, which are typically very weak and cannot be detected in the spectra of thin film.26 The results illustrated that the sawdust was functionalized with magnetic particles and 3-mercaptopropyltrimethoxysilane in the synthetic process.
To further confirm the successful modifying the magnetic sawdust with mercaptopropyl silica coating, the XPS spectra of the TF-MS was measured. Fig. 4a shows that the characteristic signals for C, O, Fe, S and Si elements are clearly observed in the XPS survey spectrum of TF-MS. The peaks of Si2s, Si2p and S2s belonged to the layer of mercaptopropyl silica (HSCH2CH2CH2Si–) could be found at 102.2 eV, 154.3 eV and 165.3 eV, respectively.27 These suggested that the silane coating was successfully modified on the surface of MS. Fig. 4b illustrates the high-resolution XPS spectra of 2p Fe. The Fe2p level with binding energies of 711.7 eV and 724.2 eV closely correspond to Fe2p3/2 and Fe2p1/2 spin–orbit peaks of γ-Fe2O3.28 The satellite peak at 719.2 eV also proves the presence of Fe3+.
For further clarifying the crystal structure and phase purity of the produces, the XRD patterns of the untreated sawdust, MS and TF-MS were measured, as shown in Fig. 5. The cellulose characteristic peaks could be seen at around 15.5° and 22.0° for both the untreated sawdust, MS and TF-MS. The additional diffraction peaks of MS and TF-MS at around 30.2°, 35.6°, 43.1°, 53.4°, 57.2° and 62.7° are corresponding to the diffractions of the (220), (311), (400), (422), (511) and (440) planes of γ-Fe2O3 (JCPDS no. 39-1346), respectively. The absence of the other peaks of TF-MS suggested that the 3-mercaptopropyltrimethoxysilane modification does not result in the phase change of γ-Fe2O3.
The introduction of magnetic γ-Fe2O3 and mercaptopropyl silica into sawdust is also confirmed by the results of TGA, as shown in Fig. 6. A small weight loss of about 5 wt% around 100 °C is due to the evaporation of adsorbed water. For the untreated sawdust, the obvious weight loss (about 60 wt%) in the temperature range from 250–320 °C is attributed to the oxidation and pyrolysis of cellulose, and the further weight loss (about 30 wt%) in the temperature range from 320–420 °C corresponded to the degradation of lignin. For the MS, there is still a weight loss of about 40 wt% and 30 wt% in the temperature range from 250–320 °C and 320–420 °C, respectively, indicating the degradation of sawdust components. However, more residues resulted after flaming, indicated some incombustible iron oxides (about 17 wt%) were precipitated on the sawdust substrate. As compared with MS, the residues of TF-MS increased weight for approximately 2 wt%, corresponding to the further modification of 3-mercaptopropyltrimethoxysilane.
Magnetic adsorbent has been attracted increasing attentions because they could be easily separated under a magnetic field. Such magnetic separation is essential to improve the operation efficiency and reduced the cost during wastewater treatment.29 Fig. 7 shows the magnetic hysteresis loop of the untreated sawdust, MS and TF-MS at room temperature. The untreated sawdust exhibit diamagnetic behavior at room temperature and the magnetization saturation value for sawdust obtained at room temperature is 0 emu g−1. However, MS and TF-MS show typical superparamagnetism at room temperature with no coercivity and remanence. The saturation magnetization values for MS and TF-MS are 11.58 and 7.28 emu g−1 at 2 T, respectively. It can be clearly seen that the saturation magnetization value is decreased after modification by MPTMS, which can be attributed to the formation of the nonmagnetic thiol-functionalized layer. Besides, the TF-MS can be collected by a magnet as shown in inset of Fig. 7, indicating that it can be easily recycled by an external magnetic field.
 | ||
| Fig. 7 Room-temperature hysteresis loops of the untreated sawdust, MS and TF-MS. The inset gives the photograph of TF-MS dispersed in water (left) and their response to a magnet (right). | ||
3.2. Effect of chemical pretreatment on heavy metal ions adsorption
The adsorption capacity of heavy metal ions on the untreated sawdust, magnetic sawdust and TF-MS were examined and Pb+ as a representative ion (Table 1). The results clearly show that TF-MS more effectively adsorbed Pb2+ ion than the untreated sawdust performed. This could be the superior ion exchange capacity of TF-MS compared to untreated sawdust and MS because of the increasing number of thiol groups on sawdust after grafting of 3-mercaptopropyltrimethoxysilane on sawdust. The chemical modification of sawdust made TF-MS to absorb more Pb2+ ions without an increase in COD, which was due to the release of the organic compounds such as lignin, pectin and tannin in the chemical pretreatment process.15| Adsorbent | COD (mg O2 per L) | Pb2+ adsorption |
|---|---|---|
| Untreated sawdust | 125 | 2.73 |
| MS | 55 | 4.45 |
| TF-MS | 46 | 9.62 |
3.3. Effect of pH
The adsorption capacity of an adsorbent for metal ions not only depends on the chemical and physical properties of the adsorbent, but also on the competitive adsorption of coexisting matters in aqueous solution and the hydrolysis capacity of the metal ions.30 Cu2+, Pb2+ and Cd2+ removal by TF-MS are measured in batch experiments with various pH values from 2 to 8, as shown in Fig. 8. The removal efficiency of Pb2+ is higher than that of Cu2+ and Cd2+ in the pH range from 2–7 and both of them present increasing trend with the rise of pH values. The minimum adsorption for Cu2+, Pb2+ and Cd2+ at pH 2.0 may be due to the fact that the competition of metal ions with H+ for combination with thiol groups and the strengthening protonation of the thiol groups on sawdust surface (pKa = 9.65) in lower acid solution. Besides, the metal ions have the tendency to hydrate to form M(OH)2 that has smaller effective size and higher mobility than metal ions under high pH solution, thus resulting in increased adsorption of adsorbent with the increase of the pH value.31 Therefore, the pH value of this study is fixed at 6.0 due to its positive effect.3.4. Effect of contact time
The effect of contact time on adsorption of Cu2+, Pb2+ and Cd2+ was studied. Fig. 9 showed that the adsorption rate was fast and achieved adsorption equilibrium within 20 min. It is possible that the surface coverage is relatively low in the early stage, so the heavy metal ions occupy the active surface sites rapidly. As the adsorption progress, the surface of TF-MS is occupied by metal ions gradually, and the rate uptake becomes slower and reaching saturation adsorption in the latter stage. | ||
| Fig. 9 Effect of contact time on adsorption of Cu2+, Pb2+ and Cd2+ (initial concentration: 10 mg L−1, adsorbent dose: 1 g L−1, pH 6.0). | ||
In order to show the most suitable kinetic model for Cu2+, Pb2+ and Cd2+ removal, the pseudo-first-order (eqn (2)), pseudo-second-order (eqn (3)), Elovich (eqn (4)) and intra-particles diffusion (eqn (5)) kinetic models are used to fit our experimental data. The pseudo-first-order, pseudo-second-order, Elovich and intra-particles diffusion rate equations are expressed as follows:32,33
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t
| (2) |
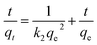 | (3) |
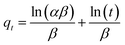 | (4) |
| qt = kintrat1/2 + c | (5) |
The parameters and the correlation coefficients (R2) for the four models are shown in Table 2. Taking the adsorption of Cu2+ by TF-MS for example, the R2 value of the pseudo-second-order model is 0.9991, which is higher than that of pseudo-first-order (0.9635), Elovich (0.9058) and intra-particles diffusion model (0.9814), revealing that the better fit to pseudo-second-order model. Besides, the adsorption capacity (qe,calc.) calculated from the pseudo-second-order model is 5.46 mg g−1, which is much closer to the experimental data (qe,exp. = 5.35 mg g−1) than the other three kinetic models. Similar phenomenon can be obtained for the adsorption of Pb2+ and Cd2+. Therefore, the obtained data are in well agreement with the pseudo-second-order kinetic model, indicating the adsorption rate of the heavy metal ions is controlled by chemical process. The order of adsorption rates was Pb2+ > Cu2+ > Cd2+.
| Kinetic models and parameters | Cu2+ C0 (mg L−1), 10.0 | Pb2+ C0 (mg L−1), 10.0 | Cd2+ C0 (mg L−1), 10.0 |
|---|---|---|---|
| qe(exp.) (mg L−1) | 5.35 | 9.62 | 3.77 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Lagergren pseudo-first-order | |||
| qe(calc.) (mg L−1) | 4.28 | 12.10 | 3.17 |
| k1 | 0.252 | 0.37 | 0.211 |
| R2 | 0.9635 | 0.9754 | 0.9831 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Pseudo-second-order equation | |||
| qe(calc.) (mg L−1) | 5.46 | 10.0 | 4.0 |
| k2 (g mg−1 min−1) | 0.189 | 0.125 | 0.178 |
| h (mg g−1 min−1) | 5.643 | 12.5 | 2.857 |
| R2 | 0.9991 | 0.9988 | 0.9993 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Elovich equation | |||
| qe(calc.) (mg L−1) | 6.20 | 11.40 | 4.05 |
| α (mg g−1 min−2) | 3.402 | 5.747 | 3.218 |
| β (g mg−1 min−1) | 0.596 | 0.314 | 1.033 |
| R2 | 0.9058 | 0.8956 | 0.9745 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Intra-particle diffusion equation | |||
| qe(calc.) (mg L−1) | 6.09 | 11.07 | 4.09 |
| kintra (mg g−1 min−1/2) | 1.574 | 2.87 | 1.05 |
| C | 0.111 | 0.17 | 0.10 |
| R2 | 0.9814 | 0.9821 | 0.9790 |
Recently, Abdel-Ghani et al. suggested that 2 h was required to reach equilibrium using chemically treated wood sawdust to adsorb 25 mg L−1 Pb2+.34 Cheng et al. pointed out that 30 min were needed for 20 mg L−1 strontium ions adsorption equilibrium using Fe3O4 modified sawdust.35 Hence, it is remarked that the TF-MS reached equilibrium within 20 min compared with other chemical modified sawdust. It is strongly believed that the thiol-groups are mainly responsible for the rapid adsorption equilibrium. The fast adsorption equilibrium provides the advantages for water treatment of highly effective and roboticized design.
3.5. Adsorption isotherm
The adsorption isotherms of Cu2+, Pb2+ and Cd2+ with corresponding Langmuir plots were showed in Fig. 10. The Langmuir adsorption model assumes that the adsorption occurs on monolayer and takes place at specific homogenous sites.2,32 It can be expressed as:
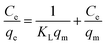 | (6) |
 | ||
| Fig. 10 Experimental metal adsorption isotherms and modeled results using Langmuir equation (initial concentration ranging from 2 to 30 mg L−1, adsorbent dose: 1 g L−1, pH 6.0, contact time: 30 min). | ||
| Langmuir model | Freundlich model | |||||
|---|---|---|---|---|---|---|
| qm (mg g−1) | KL (L mg−1) | R2 | KF | n | R2 | |
| Cu2+ | 5.49 | 10.71 | 0.9816 | 4.19 | 4.23 | 0.8949 |
| Pb2+ | 12.50 | 8 | 0.9808 | 6.75 | 2.22 | 0.9029 |
| Cd2+ | 3.80 | 13.85 | 0.9938 | 3.00 | 5.95 | 0.9821 |
The Freundlich model is applied for multilayer sorption and non-ideal sorption on heterogeneous surface sites, and can be represented in linear form as follows:
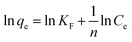 | (7) |
Taking Cu2+ for example, from Table 3, the correlation coefficients R2 of Langmuir equation is 0.9816, which is higher than that of Freundlich equation (0.8949). Therefore, the Langmuir isotherm model correlates better than the Freundlich isotherm model with the experimental data, suggesting the adsorbed Cu2+ by TF-MS is a monolayer adsorption. Similar phenomenon can be obtained for the adsorption of Pb2+ and Cd2+. According to the Langmuir equation, the adsorption capacity of Cu2+, Pb2+ and Cd2+ on TF-MS were calculated to be 5.49 mg g−1, 12.5 mg g−1 and 3.80 mg g−1, respectively. A comparison of the maximum capacity, qmax, of TF-MS with those of some other biomass materials reported in literature is given in Table S2.† Comparison of heavy metal ions adsorption on TF-MS with the literature data indicates that the adsorption capacity of TF-MS is greater than other biomass materials. Moreover, the maximum adsorption capacity ranked in the order of Pb2+ > Cu2+ > Cd2+, which is the same with the calculated qe order as expected in the kinetic studies and the one deduced from the study of the impact of pH. The accordant conclusions coming from the adsorption kinetic, isotherms and pH study indicated a differential binding capacity Pb2+ > Cu2+ > Cd2+.
3.6. Competitive adsorption
The competitive adsorption among Cu2+, Pb2+ and Cd2+ was investigated, as shown in Fig. 11. For Cu2+ and Cd2+ bi-metal solution, the efficiency of Cu2+ and Cd2+ is 71.1% and 45.7%, respectively. In Pb2+ and Cd2+ mixed solution, the removal of Pb2+ is triple of Cd2+. And in Pb2+ and Cu2+ mixed solution, the removal of Pb2+ is double of Cu2+. Obviously, in the presence of Pb2+, adsorption of Cu2+ and Cd2+ is strongly restricted. For the tri-metal solution with equal concentration of Cu2+, Pb2+ and Cd2+, the corresponding removal efficiency is 42.5%, 90.6%, and 29.1%, respectively. These indicated that the TF-MS have a preferential adsorption of Pb2+ > Cu2+ > Cd2+. It could be explained by Pearson's theory that soft ligands like the thiol group on the surface of sawdust are soft base, which can form a highly polarizable donor centres, thereby have the capable of interacting and strong affinity with low-lying orbitals of soft acid, and Pb is considered to be softer than Cu and Cd.31,36 | ||
| Fig. 11 Competitive adsorption of multi-metal solution (adsorbent dose: 1 g L−1, pH: 6.0, contact time: 30 min). | ||
3.7. Stability and regeneration
Stability in different conditions is important for the practical application of the adsorbent. As for the degree of acid and alkali resistance, the results are showed in Table 4. The magnetization values of TF-MS after 2 M HCl treatment is still maintained at 4.41 emu g−1, which is only decreased 2.87 emu g−1 compared with the untreated TF-MS. This value is smaller than that reported in many studies,37,38 indicating the good magnetization stability of TF-MS. The magnetization values of TF-MS increased slightly after alkali treatment. It is possibly due to the chemical reaction between the silane layer and NaOH, which reduced the content of nonmagnetic silane in TF-MS and increased the magnetic values. Moreover, the magnetization values of all treated TF-MS are strong enough for separation in 1 min. As for the removal efficiency of treated TF-MS, it can observe that the removal efficiency of Pb2+ decreased after acid treatment, which is caused by the protonation of TF-MS adsorbent in the process of acid treatment. Although the treated adsorbent was washed repeatedly to reach neutral for reuse, some H+ occupied the binding sites on the adsorbent surface was irrevocable, which led to the decrease of removal efficiency. On the contrary, the removal efficiency increased after alkali treatment. It is believed that the adsorbent was deprotonated and some hydroxyl groups might form on the TF-MS surface to chelate metal ions, resulting in the subsequent increase of removal efficiency. Therefore, it can be expected that TF-MS can be used in wastewater even under extreme condition.| HCl | NaOH | Untreated | ||||||
|---|---|---|---|---|---|---|---|---|
| Concentration (mol L−1) | 0.1 | 1 | 2 | 0.1 | 1 | 2 | 5 | |
| Magnetization (emu g−1) | 7.07 | 6.57 | 4.41 | 8.08 | 8.12 | 9.09 | 10.58 | 7.28 |
| Removal% | 56.5 | 49.1 | 43.3 | 74.5 | 75.9 | 77.3 | 78.9 | 73.6 |
The regeneration of TF-MS is surveyed by eluted solution of 1 M HCl under sonication for 30 min. Fig. 12 shows the removal efficiency of Pb2+ over four successive adsorption–desorption cycles. It is observed that approximate 96% removal efficiency is reached in the first cycle. Even though the efficiency decreased with the increasing of cycle, over 87% efficiency was obtained in the further adsorption–desorption cycle.
 | ||
| Fig. 12 Pb2+ adsorption on regenerated TF-MS adsorbent with four adsorption–regeneration cycles (initial concentration: 10 mg L−1, adsorbent dose: 1 g L−1, pH 6.0). | ||
4. Conclusion
Thiol-functionalized magnetic sawdust adsorbent was prepared for removal of heavy metals. Such prepared adsorbent possessed the superparamagnetic character, which make it an effective and convenient adsorbent for heavy metal removal. The adsorption of Cu2+, Pb2+ and Cd2+ was well modeled by pseudo-second-order model and Langmuir adsorption isotherm, and the adsorbent presented a preferential binding capacity of Pb2+ > Cu2+ > Cd2+. Besides, the adsorbent possess excellent stability in strong acid and alkaline condition, and could be used repeatedly by effective regeneration using acid solution. Coupled with the advantages of fast, easy separation, environmental friendliness and appropriate adsorption capacity for heavy metal ions, the approach presented may provide further routes for the development of efficient and recyclable biosorbent.Acknowledgements
This work was financially supported by The National Natural Science Foundation of China (grant no. 31470584).References
- W. Yantasee, C. L. Warner, T. Sangvanich, R. S. Addleman, T. G. Carter, R. J. Wiacek and M. G. Warner, Removal of heavy metals from aqueous systems with thiol functionalized superparamagnetic nanoparticles, Environ. Sci. Technol., 2007, 41, 5114–5119 CrossRef CAS PubMed.
- Y. M. Hao, C. Man and Z. B. Hu, Effective removal of Cu(II) ions from aqueous solution by amino-functionalized magnetic nanoparticles, J. Hazard. Mater., 2010, 184, 392–399 CrossRef CAS PubMed.
- S. E. Bailey, T. J. Olin, R. M. Bricka and D. D. Adrian, A review of potentially low-cost sorbents for heavy metals, Water Res., 1999, 33, 2469–2479 CrossRef CAS.
- X. Liu, Q. Hu, Z. Fang, X. Zhang and B. Zhang, Magnetic chitosan nanocomposites: a useful recyclable tool for heavy metal ion removal, Langmuir, 2008, 25, 3–8 CrossRef PubMed.
- S. Wang, K. Wang, C. Dai, H. Shi and J. Li, Adsorption of Pb2+ on amino-functionalized core–shell magnetic mesoporous SBA-15 silica composite, Chem. Eng. J., 2015, 262, 897–903 CrossRef CAS.
- M. Ozmen, K. Can, G. Arslan, A. Tor, Y. Cengeloglu and M. Ersoz, Adsorption of Cu(II) from aqueous solution by using modified Fe3O4 magnetic nanoparticles, Desalination, 2010, 254, 162–169 CrossRef CAS.
- W. W. Ngah and M. A. K. M. Hanafiah, Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: a review, Bioresour. Technol., 2008, 99, 3935–3948 CrossRef PubMed.
- T. W. Tee and A. R. M. Khan, Removal of lead, cadmium and zinc by waste tea leaves, Environ. Technol., 1988, 9, 1223–1232 CrossRef CAS.
- S. Liang, X. Guo, N. Feng and Q. Tian, Application of orange peel xanthate for the adsorption of Pb2+ from aqueous solutions, J. Hazard. Mater., 2009, 170, 425–429 CrossRef CAS PubMed.
- Z. Y. Yao, J. H. Qi and L. H. Wang, Equilibrium, kinetic and thermodynamic studies on the biosorption of Cu(II) onto chestnut shell, J. Hazard. Mater., 2010, 174, 137–143 CrossRef CAS PubMed.
- O. Karnitz, L. V. A. Gurgel, J. C. P. De Melo, V. R. Botaro, T. M. S. Melo, R. P. de Freitas Gil and L. F. Gil, Adsorption of heavy metal ion from aqueous single metal solution by chemically modified sugarcane bagasse, Bioresour. Technol., 2007, 98, 1291–1297 CrossRef CAS PubMed.
- H. Chen, J. Zhao, G. Dai, J. Wu and H. Yan, Adsorption characteristics of Pb(II) from aqueous solution onto a natural biosorbent, fallen Cinnamomum camphora leaves, Desalination, 2010, 262, 174–182 CrossRef CAS.
- A. K. Bhattacharya, S. N. Mandal and S. K. Das, Adsorption of Zn(II) from aqueous solution by using different adsorbents, Chem. Eng. J., 2006, 123, 43–51 CrossRef CAS.
- M. Sciban, M. Klasnja and B. Skrbic, Modified hardwood sawdust as adsorbent of heavy metal ions from water, Wood Sci. Technol., 2006, 40, 217–227 CrossRef CAS.
- N. Feng, X. Guo and S. Liang, Adsorption study of copper(II) by chemically modified orange peel, J. Hazard. Mater., 2009, 164, 1286–1292 CrossRef CAS PubMed.
- R. S. Bai and T. E. Abraham, Studies on enhancement of Cr(VI) biosorption by chemically modified biomass of Rhizopus nigricans, Water Res., 2002, 36, 1224–1236 CrossRef CAS PubMed.
- M. Najafi, R. Rostamian and A. A. Rafati, Chemically modified silica gel with thiol group as an adsorbent for retention of some toxic soft metal ions from water and industrial effluent, Chem. Eng. J., 2011, 168, 426–432 CrossRef CAS.
- R. Rostamian, M. Najafi and A. A. Rafati, Synthesis and characterization of thiol-functionalized silica nano hollow sphere as a novel adsorbent for removal of poisonous heavy metal ions from water: Kinetics, isotherms and error analysis, Chem. Eng. J., 2011, 171, 1004–1011 CrossRef CAS.
- V. Merk, M. Chanana, N. Gierlinger, A. M. Hirt and I. Burgert, Hybrid wood materials with magnetic anisotropy dictated by the hierarchical cell structure, ACS Appl. Mater. Interfaces, 2014, 6, 9760–9767 CAS.
- S. Trey, R. T. Olsson, V. Ström, L. Berglund and M. Johansson, Controlled deposition of magnetic particles within the 3-D template of wood: making use of the natural hierarchical structure of wood, RSC Adv., 2014, 4, 35678–35685 RSC.
- W. Gan, L. Gao, X. Zhan and J. Li, Hydrothermal synthesis of magnetic wood composites and improved wood properties by precipitation with CoFe2O4/hydroxyapatite, RSC Adv., 2015, 5, 45919–45927 RSC.
- M. Šćiban, B. Radetić, Ž. Kevrešan and M. Klašnja, Adsorption of heavy metals from electroplating wastewater by wood sawdust, Bioresour. Technol., 2007, 98, 402–409 CrossRef PubMed.
- N. Gierlinger, L. Goswami, M. Schmidt, I. Burgert, C. Coutand, T. Rogge and M. Schwanninger, In situ FT-IR microscopic study on enzymatic treatment of poplar wood cross-sections, Biomacromolecules, 2008, 9, 2194–2201 CrossRef CAS PubMed.
- R. D. Waldron, Infrared spectra of ferrites, Phys. Rev., 1955, 99, 1727 CrossRef CAS.
- O. Hakami, Y. Zhang and C. J. Banks, Thiol-functionalised mesoporous silica-coated magnetite nanoparticles for high efficiency removal and recovery of Hg from water, Water Res., 2012, 46, 3913–3922 CrossRef CAS PubMed.
- A. Walcarius and C. Delacôte, Mercury(II) binding to thiolfunctionalized mesoporous silicas: critical effect of pH and sorbent properties on capacity and selectivity, Anal. Chim. Acta, 2005, 547, 3–13 CrossRef CAS.
- S. Li, X. Yue, Y. Jing, S. Bai and Z. Dai, Fabrication of zonal thiol-functionalized silica nanofibers for removal of heavy metal ions from wastewater, Colloids Surf., A, 2011, 380, 229–233 CrossRef CAS.
- C. Wan and J. Li, Facile synthesis of well-dispersed superparamagnetic γ-Fe2O3 nanoparticles encapsulated in three-dimensional architectures of cellulose aerogels and their applications for Cr(VI) removal from contaminated water, ACS Sustainable Chem. Eng., 2015, 3, 2142–2152 CrossRef CAS.
- M. Hua, S. Zhang, B. Pan, W. Zhang, L. Lv and Q. Zhang, Heavy metal removal from water/wastewater by nanosized metal oxides: a review, J. Hazard. Mater., 2012, 211, 317–331 CrossRef PubMed.
- C. Zhang, J. Sui, J. Li, Y. Tang and W. Cai, Efficient removal of heavy metal ions by thiol-functionalized superparamagnetic carbon nanotubes, Chem. Eng. J., 2012, 210, 45–52 CrossRef CAS.
- G. Li, Z. Zhao, J. Liu and G. Jiang, Effective heavy metal removal from aqueous systems by thiol functionalized magnetic mesoporous silica, J. Hazard. Mater., 2011, 192, 277–283 CAS.
- Y. Tan, M. Chen and Y. Hao, High efficient removal of Pb(II) by amino-functionalized Fe3O4 magnetic nano-particles, Chem. Eng. J., 2012, 191, 104–111 CrossRef CAS.
- M. Ozmen, K. Can, I. Akin, G. Arslan, A. Tor, Y. Cengeloglu and M. Ersoz, Surface modification of glass beads with glutaraldehyde: Characterization and their adsorption property for metal ions, J. Hazard. Mater., 2009, 171, 594–600 CrossRef CAS PubMed.
- N. T. Abdel-Ghani, G. A. El-Chaghaby and F. S. Helal, Simultaneous removal of aluminum, iron, copper, zinc, and lead from aqueous solution using raw and chemically treated African beech wood sawdust, Desalin. Water Treat., 2013, 51, 3558–3575 CrossRef CAS.
- Z. Cheng, Z. Gao, W. Ma, Q. Sun, B. Wang and X. Wang, Preparation of magnetic Fe3O4 particles modified sawdust as the adsorbent to remove strontium ions, Chem. Eng. J., 2012, 209, 451–457 CrossRef CAS.
- R. G. Pearson, Hard and soft acids and bases, J. Am. Chem. Soc., 1963, 85, 3533–3539 CrossRef CAS.
- Y. Pang, G. Zeng, L. Tang, Y. Zhang, Y. Liu, X. Lei and G. Xie, PEI-grafted magnetic porous powder for highly effective adsorption of heavy metal ions, Desalination, 2011, 281, 278–284 CrossRef CAS.
- J. Wang, S. Zheng, Y. Shao, J. Liu, Z. Xu and D. Zhu, Amino-functionalized Fe3O4@SiO2 core–shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal, J. Colloid Interface Sci., 2010, 349, 293–299 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra02285e |
| This journal is © The Royal Society of Chemistry 2016 |







