Mass spectrometry based chemical imaging of foods
Abstract
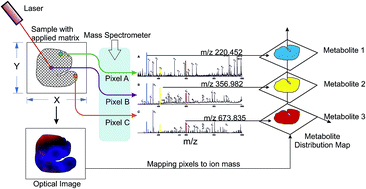
Chemical imaging based on mass spectrometry is an emerging technology which has opened opportunities for fundamental research in food science. The ability to quantitatively determine the spatial distribution of several chemical entities simultaneously makes it a method of choice for chemical characterization of plant materials and foods. In this review, an overview of the ionization methods is presented, followed by a discussion of the current state of the art in mass spectrometry imaging (MSI) of relevance to practices in food research. The known applications, analytical challenges, and future research potential are highlighted. MSI has been successfully utilized to a variable degree for obtaining information about spatial distribution of a variety of molecules and metabolites in foods. It allows visualizing the spatial molecular maps without extraction, purification, separation, or labelling of foods. The method is likely to find wider adoption with developments in ionization sources and inter-laboratory collaborations.


 Please wait while we load your content...
Please wait while we load your content...