Self-cleaning pH/thermo-responsive cotton fabric with smart-control and reusable functions for oil/water separation†
J. D. Wuabc,
C. Zhangc,
D. J. Jianga,
S. F. Zhaoa,
Y. L. Jiangc,
G. Q. Caiabc and
J. P. Wang*abc
aMOE Key Laboratory of Advanced Textile Materials & Manufacturing Technology, Zhejiang Sci-Tech University, Hangzhou 310018, China. E-mail: jipingwanghz@gmail.com
bNational Base for International Science & Technology Cooperation in Textiles and Consumer-Goods Chemistry, Zhejiang Sci-Tech University, Hangzhou 310018, China
cMOE Engineering Research Center for Eco-Dyeing and Finishing of Textiles, Zhejiang Sci-Tech University, Hangzhou 310018, China
First published on 22nd February 2016
Abstract
Nowadays, the environmental problem caused by oily wastewater discharge and crude oil leakage has attracted worldwide attention. Recently developed technologies for oil/water separation have been based mainly on materials with special wettability. In this work, a self-cleaning system with smart-control and reusable functions for oil/water separation has been built on cotton fabric via surface-initiated atom transfer radical polymerization (ATRP) of 2-dimethylaminoethyl methacrylate (DMAEMA). The fabric showed high pH- and thermo-responsibility, which was strongly dependent on the pDMAEMA grafting ratio. And by modulating pH or temperature, the fabric switched from superhydrophilic (WCA ∼ 0°) to highly hydrophobic (WCA ∼ 130°), and therefore, were suitable both for the separation of water-rich or oil-rich oil/water mixtures as a type of adsorbent material. The fabric could adsorb oil over 4 times its own weight, and reversibly release it in acidic water, making the fabric easily recyclable.
Introduction
Along with the sustained and rapid development of economy, a large amount of oily wastewater is discharged from industry. It has brought great damage to the environment since the float oil layer can cover water and cause a large number of aquatic organisms to die from lack of oxygen. Furthermore, the polycyclic aromatic hydrocarbons in oil slick, which is toxic and carcinogenic, could be taken into the human body through the food chain. Therefore, oil/water separation has been an important and urgent issue, which has attracted the extensive concern of government and scientists.Various approaches have been used in oil/water separation such as gravity separation, centrifugation, ultrasonic separation, air flotation, electric field, coagulation and biological treatment.1 Scientists have developed an efficient method for oil/water separation by using materials with special wettability.2–4 Liu et al. used sol–gel method to generate silicon nanoparticles on raw cotton, which were then modified by octadecyltrichlorosilane (OTS).5 It removed oil from water by selectively adsorbing oil due to its oil-loving and water-repelling properties. A superhydrophobic cotton textile was fabricated by coating ZnO nanoparticles and polystyrene.6 This kind of textile could be used as an effective filtration material for oil/water mixtures. However, these materials, especially the hydrophobic ones, are prone to be contaminated by low-surface-energy oils, which may lead to the loss of their special surface wettability and separation performance.7,8
In order to meet this challenge, new strategies for separation materials with long-term efficiency and self-cleaning is highly desirable.9 Zhang et al. created a type of self-cleaning mesh by the layer-by-layer (LBL) assembly of sodium silicate and TiO2 nanoparticles, which enabled removal of the organic contaminants by ultraviolet (UV) illumination.10 Materials with underwater superoleophobicity were also developed to solve the above-mentioned problem.11,12 For example, polyamide was crosslinked onto stainless steel meshes and formed a layer of hydrogel on the surface.7 Thus, the material allowed water to pass, while preventing the membrane from fouling by oil. It is anticipated that smart materials are more desirable for highly controllable and sustainable oil/water separation, since they can reversibly switch from superhydrophilic to superhydrophobic in response to changes in the external environment, such as temperature,13,14 pH,15 light,16–18 forces,19 etc. For example, Zhang et al. grafted the poly(2-vinylpyridine-b-dimethylsiloxane) block copolymer onto polyurethane sponge and it could be applied to reversibly oil capture and release in water by changing the pH of the aqueous media.20 The material can be regenerated and recycled many times due to its smart oil-spill cleanup ability.
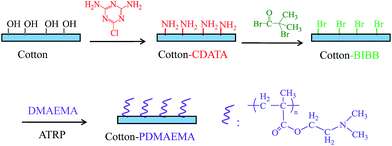
At present, most studies have focused on the application of single-responsive materials, whereas, the multi-responsive ones are believed to be more intelligent to adapt to complex environmental changes.21,22 In this work, poly(2-dimethylaminoethyl methacrylate) (pDAMEMA) was grafted on to cotton fabric via a surface-initiated ATRP technique. Scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), infrared (IR) and contact angle (CA) measurement were employed to characterize the surface morphology, chemical composition and surface wettability, respectively. Cotton is a type of natural fiber with many excellent characteristics, including low cost, good softness and flexibility, ease of transport, etc. Moreover, it is a sort of porous matrix and possesses a comparative micro-roughness, which is of high stability and can favor the generation of a surface with special wettability according to the Wenzel theory.23,24 Without the integration of inorganic particles to construct nanostructures, the pDMAEMA-grafted fabric showed high temperature and pH responsive performance, and highly effective oil/water separations could be achieved. The oil/water adsorption and releasing behaviors of pDMAEMA-grafted fabric were investigated.
Experimental
Materials
2-Chloro-4,6-diamino-1,3,5-triazine (CDATA, 99%), bromoisobutyryl bromide (BIBB, 99%), 2-dimethylaminoethyl methacrylate (DMAEMA, 99%), N,N,N′,N′,N′′-pentamethyldiethylenetriamine (PMDETA, 99%) and copper(I) bromide (CuBr, A. R.) were purchased from Aladdin Industrial Co. Ltd. DMAEMA was purified by vacuum distillation before use. Sodium carbonate (Na2CO3), sodium sulphate (Na2SO4), dichloromethane, triethylamine (TEA), acetone, ethanol, methanol, acetic acid, hydrochloric acid (36%) and sodium hydroxide were bought from Sinopharm Chemical Reagent, Ltd. Co. and were used as received. Reactive red 195 and oil red was purchased from Yashilin Ltd. Co. and Sigma, respectively. The corn oil was purchased from the local supermarket. A cotton fabric (plain & woven) was purchased from Zhejiang Furun Printing & Dyeing Co., Ltd. It was used after being washed with a surfactant solution twice, rinsed with a large amount of water and dried in an oven at 80 °C for 6 h.pDMAEMA grafting by surface-initiated ATRP
The cotton fabric was immersed into an aqueous solution with 10% 2-chloro-4,6-diamino-1,3,5-triazine (CDATA), 10 g L−1 sodium carbonate (Na2CO3) and sodium sulphate (Na2SO4). The liquor ratio of the cotton fabric to solutions was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. The reaction was taken at 80 °C for 6 h. Then the sample was rinsed with water and dried under a vacuum oven at 80 °C for 6 h.
50. The reaction was taken at 80 °C for 6 h. Then the sample was rinsed with water and dried under a vacuum oven at 80 °C for 6 h.
The cotton-CDTAZ fabric was incubated in a solution of dichloromethane and TEA (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), followed by a drop-wise addition of a dichloromethane solution containing 5% (v/v) BIBB. The reaction proceeded at 0 °C for 2 h and then continued at room temperature for another 12 h. The fabric was washed with acetone, ethanol and water in sequence, and then dried at 80 °C for 6 h.
1, v/v), followed by a drop-wise addition of a dichloromethane solution containing 5% (v/v) BIBB. The reaction proceeded at 0 °C for 2 h and then continued at room temperature for another 12 h. The fabric was washed with acetone, ethanol and water in sequence, and then dried at 80 °C for 6 h.
A solution of methanol/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 10 mL), PMDETA (0.4 mL), CuBr (0.1 g) and DMAEMA (2.0 mL) were degassed under nitrogen flow for about 2 h. It was added to a tube which contained a cotton-BIBB fabric and was previously evacuated and replaced with nitrogen. The polymerization was carried out at 60 °C and terminated by oxygen. Finally, the cotton-pDMAEMA fabric was washed with a copious amount of water and dried in an oven at 60 °C for 6 h.
1 v/v, 10 mL), PMDETA (0.4 mL), CuBr (0.1 g) and DMAEMA (2.0 mL) were degassed under nitrogen flow for about 2 h. It was added to a tube which contained a cotton-BIBB fabric and was previously evacuated and replaced with nitrogen. The polymerization was carried out at 60 °C and terminated by oxygen. Finally, the cotton-pDMAEMA fabric was washed with a copious amount of water and dried in an oven at 60 °C for 6 h.
Oil/water separation experiments
The fabric was pretreated with solutions of different pH (1, 3, 5, 7, 9, 11, 13), followed by being dried in the oven at 60 °C for 2 h. Then they were immersed into a corn oil/water mixture for the separation experiment. In a water-rich mixture, oil was stained by oil red. And in an oil-rich mixture, water was stained by reactive red 195. The temperature of the mixture was controlled by heating. The oil desorption experiment was performed by putting the oil-containing fabric into an acidic aqueous solution (pH = 1, 25 °C).In order to measure the maximum adsorption capacity of fabric for oil, the experiment was carried out in pure corn oil.5 In detail, 1 g fabric pretreated with basic solution (pH = 13) was immersed in oil for 1 min. The oil in fabric was drained for 30 s before being weighed. The adsorption capacity was defined as the increase in weight of the fabric after adsorption divided by the initial weight of the dry fabric. The final oil adsorption capacity was the average value of 5 experiments. The water adsorption capacity was measured by the same method.
Characterization
The surface chemistry was analyzed by infrared under attenuated total reflection (IR-ATR) mode (AVATAR 5700, Nicolet, America) and X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific K-Alpha*, America). The XPS spectrum was differentiated and fitted using XPS peak software. The binding energy (B.E) was corrected by setting the C 1s peak (C–C) at 284.8 eV. The surface morphology of the fibers was studied via a scanning electron microscope (SEM, JSM-5610Lv, Japan).The contact angle and wicking time was measured by a contact angle analyzer (DSA 20, KRUSS, Germany). The samples were pretreated by aqueous solution of different pH and then dried in an oven. To study the contact angle under different temperatures, a thermal platform mounted with a water circulation system was used. The temperature of the heating stage is preset, followed by placing the fabric on the stage and balancing for 10 min. A droplet of water (2 μL) was dipped onto fabric and the contact angle at 1 s was calculated. To study the underwater oil contact angle (OCA), the sample was placed underwater and corn oil was injected into water. The oil droplet contacted the undersurface of the textile due to its lower density compared to water and the OCA was measured.
Results and discussion
Surface chemistry
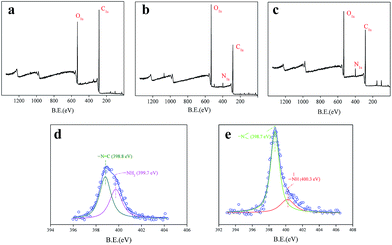
A dye intermediate was used to introduce amino groups onto cotton. It is a simple and efficient method, which has been reported in our previous work.25 The produced amino groups were used as a grafting site for functional polymers. In this work, pDMAEMA was grafted on to cotton via surface-initiated ATRP technology (Fig. 1). XPS was employed to characterize the surface chemistry of cotton fabric to confirm successful modification. No nitrogen was detected on the cotton surface, while a N 1s peak appeared on the spectrum of CDATA-modified cotton (Fig. 2a and b). 2.2% N was found on the CDATA-modified surface while 4.5% on the pDMAEMA-grafted surface (Fig. 2b and c), which means pDMAEMA has been successfully grafted onto cotton. In the nitrogen spectrum of the CDATA-modified surface, the peaks at 398.8 eV and 399.7 eV were attributed to –![[N with combining low line]](https://www.rsc.org/images/entities/char_004e_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and –
C and –![[N with combining low line]](https://www.rsc.org/images/entities/char_004e_0332.gif) H2, respectively (Fig. 2d). The relative peak area ratio approached 3
H2, respectively (Fig. 2d). The relative peak area ratio approached 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, which is the atom ratio of the two nitrogens in the CDATA molecule. For a pDMAEMA-grafted cotton surface, the peak shifted to 400.3 eV since the amino groups were converted to amide groups (Fig. 2e). The peak at 398.7 eV, which originated from the nitrogen in a pDMAEMA molecule, was much stronger than the peak of the amide groups. It was suggested that the molecular weight of pDMAEMA grafted on to the cotton was high.
2, which is the atom ratio of the two nitrogens in the CDATA molecule. For a pDMAEMA-grafted cotton surface, the peak shifted to 400.3 eV since the amino groups were converted to amide groups (Fig. 2e). The peak at 398.7 eV, which originated from the nitrogen in a pDMAEMA molecule, was much stronger than the peak of the amide groups. It was suggested that the molecular weight of pDMAEMA grafted on to the cotton was high.
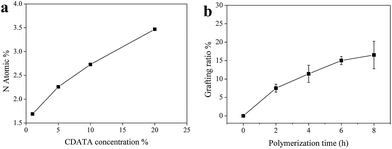
The functions of surface-grafted polymer, for example, the pH-responsibility, depend on the chain density and length. Through the CDATA treatment method, the density of the amino groups could be well-controlled. The atomic percentage of nitrogen on CDATA-treated fabric was measured by XPS (Fig. 3a). As the concentration of CDATA solution increased, the content of nitrogen increased, that is the density of grafting sites for polymer chains increased. In this work, the grafting density was controlled to the same level by treating cotton with 10% CDATA. On the other hand, the grafting ratio of pDMAEMA could be modulated by polymerization time. As the polymerization time increased, the grafting ratio increased (Fig. 3b). The polymerization rate slightly decreased with polymerization time, which may be due to the steric hindrance of neighboring polymer chains. In our case, the grafting ratio was correlated with pDMAEMA molecular weight.
The FTIR spectra of pDMAEMA grafted cotton are illustrated in Fig. 4. The peaks at 2890 cm−1 attributed to methyl and methylene groups. The peaks of carbonyl groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in DMAEMA molecules appeared at 1725 cm−1, while the peaks at 3300 cm−1, 1639 cm−1 and 1108 cm−1 corresponded to O–H stretching, O–H bending and C–O stretching vibrations in cellulose molecules, respectively. With the increase of the pDMAEMA grafting ratio, the characteristic peaks of cotton weakened, while the peaks of carbonyl groups (1725 cm−1) were strengthened. It suggested that cotton fibers were covered by an increasing amount of polymer.
O) in DMAEMA molecules appeared at 1725 cm−1, while the peaks at 3300 cm−1, 1639 cm−1 and 1108 cm−1 corresponded to O–H stretching, O–H bending and C–O stretching vibrations in cellulose molecules, respectively. With the increase of the pDMAEMA grafting ratio, the characteristic peaks of cotton weakened, while the peaks of carbonyl groups (1725 cm−1) were strengthened. It suggested that cotton fibers were covered by an increasing amount of polymer.
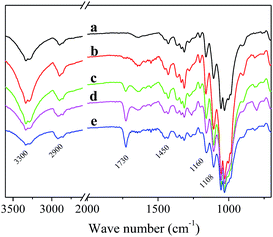 | ||
| Fig. 4 The IR spectrum of different cotton: (a) raw cotton, (b–e) pDMAEMA-grafted fabric with different grafting ratios (b: 2.3%; c: 5.3%; d: 9.4%; e: 12.4%). | ||
Surface morphology
The morphology of pDMAEMA grafted cotton fabric was observed using SEM. The natural cotton fibers were oblate with orderly aligned wrinkle structures (Fig. 5a). The diameter of cotton fiber was about 30 μm. Micro-structures were found on natural cotton fibers, which were then covered by polymeric film after pDMAEMA grafting (Fig. 5b and c). When the grafting ratio was about 10%, the fibers became smooth because of the thick and homogenous polymer layer. However, the fabric with the higher growing weight showed a much rougher surface, which may be caused by the uncontrollable and inhomogeneous polymerization in the late period. | ||
| Fig. 5 The morphology of (a) raw cotton and pDMAEMA-grafted cotton with grafting ratios (b) 5.3% and (c) 12.4%. | ||
Surface wettability and pH- and thermo-responsibility
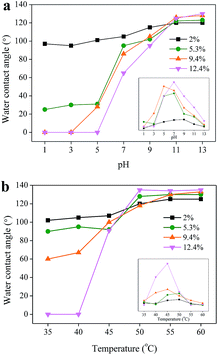
The water contact angle of raw cotton fabric was about 100°, while the fabric showed both pH- and thermo-responsibility after pDMAEMA grafting. By either increasing pH or temperature, the pDMAEMA-grafted cotton fabric changed from hydrophilic to hydrophobic (Fig. 6a and b). In detail, at low pH, the water contact angle of fabric was 0° (at 1 s), which then increased as the pH value rose. An abrupt transition was found in the range of 5–11 (Fig. 6a). Shown by the derivation curve (inserted figure), the sharpest transition, that is, the transition pH value appeared around 7. As the grafting ratio of pDMAEMA on cotton fabric increased, the transition of surface wettability became more significant. For example, when the grafting ratio was about 2%, the contact angle changed from about 100° (pH = 1) to 110° (pH = 13). But for cotton fabric with 12.4% pDMAEMA, the contact angle changed largely from 0° to 130°.The surface wettability of pDMAEMA-grafted cotton fabric could also be controlled by the environment temperature. The sample was incubated in water (pH = 6.5) previously before contact angle measurement. When elevating the temperature, the contact angle also showed an abrupt transition from hydrophilic to hydrophobic (Fig. 6b). By calculating the derivation, it was learned that the transition temperature was around 45 °C. Similar to pH-responsibility, fabrics with a larger amount of pDMAEMA showed better thermo-responsibility.
pDMAEMA has pH and temperature responsibilities due to the presence of tertiary amine and hydrophobic groups.26,27 The pKa of pDMAEMA is about 7.0–7.5 and therefore nitrogen in the DMAEMA unit accepts a proton and forms a cation unit (NH3+ forms) at low pH.28 The surface with high positive charges forms a strong association with water molecules, making itself hydrophilic. With increasing pH, pDMAEMA deprotonates becoming uncharged chains (NH2 forms), collapsing on the surface because of the hydrophobic force among them, resulting in a hydrophobic surface. pDMAEMA also is a temperature-sensitive polymer. It could associate with water molecules at low temperature caused by intermolecular hydrogen bonds. When the temperature increases, the intramolecular interaction between the pDMAEMA chains becomes considerable and finally suppresses the interactions with water at temperatures above 50 °C. Both the pH- and thermo-responsibility showed a strong dependence on the grafting ratio of pDMAEMA. The responsive behavior relied on the number of DMAEMA moiety units. pDMAEMA chains with a higher molecular weight could provide a higher density of surface charge at low pH and a longer hydrophobic backbone aggregated on the surface at high pH. Similarly, longer pDMAEMA chains could provide more sites for intermolecular/intramolecular interactions below/above the transition temperature. Therefore, cotton fabric with a higher pDMAEMA grafting ratio showed an efficient responsibility to the pH and temperature variation, which made it more suitable for application. It should be noted that the transition pH value was not clearly correlated with the molecular weight of pDMAEMA, while the transition temperature slightly shifted to a lower value with increasing pDMAEMA grafting ratio. The reason may be that the temperature triggered transition behavior is more dependent on the intramolecular interactions. It is easier for longer polymer chains to form intramolecular associations and favor the collapse of chains.
Oil/water separation
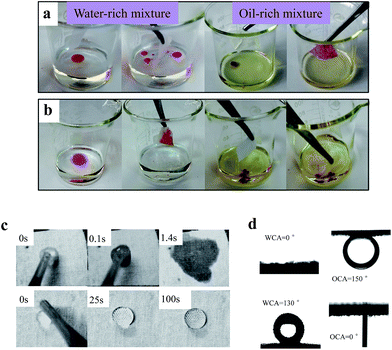
Textile is suitable for practical oil absorption in oil spill accidents owing to their excellent properties such as high absorption capability and low cost.29 In this work, the separation performance of pDMAEMA-grafted cotton fabric were studied by immersing the fabric into a corn oil/water mixture for 1 min and then taking them out. Raw cotton fabric could both adsorb oil and water in the mixtures, whereas the pDMAEMA-grafted cotton showed excellent adsorption capacity and selectivity due to its surface property and high porous structure. The hydrophilic pDMAEMA-grafted cotton (pH 1, 25 °C) allowed water uptake while repelling oil completely (Fig. 7a). In this way, water could be removed from oil. In contrast, the hydrophobic pDMAEMA-grafted cotton (pH 13, 25 °C) preferred to take in oil rather than water (Fig. 7b). It could be used to separate oil from water. The separation efficiency was high since no visible oil existed in the mixture after separation.The high oil or water selectivity of pDMAEMA-grafted cotton compared to cotton was due to the super anti-wetting or super wetting properties (Fig. 7c and d). The water spreading process on fabric at two states (pH 1, 25 °C and pH 13, 25 °C) was recorded by a high-speed camera (Fig. 7c). The water droplet permeated into the hydrophilic fabric immediately on contact with the surface, while the water droplet sat on the hydrophobic fabric for a long time. As the pH increased, the wetting time (the time for water droplets spreading on fabric) increased from nearly 0.3 s to about 139 s. The water and underwater oil contact angles were also measured (Fig. 7d). The hydrophilic fabric could be wetted by water very quickly and the WCA is 0°. It repelled the oil totally in water and the OCA was about 150°. In contrast, the fabric at pH 13 was highly hydrophobic with a WCA about 130° and underwater OCA 0°. Using cotton fabric as sorbent, liquid can be taken into the matrix and trapped in the holes among the fibers. It is superior for water to contact fabric with low surface energy and the polymers associate with the water to form a hydration layer on the surface, which could prevent oil coming into the holes.4 In the same way, the hydrophobic fabric could repel water by quickly adsorbing the oil and forming an oil layer. This may be the reason for the high water/oil selectivity of pDMAEMA-grafted cotton fabric at different conditions.
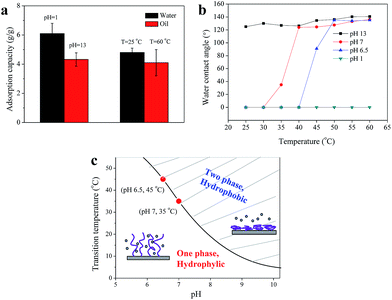
The maximum adsorption capacity of pDMAEMA modified fabric at different states was shown in Fig. 8a. At low pH (pH = 1) or low temperature (T = 25 °C), the fabric was hydrophilic and adsorbed water, whereas it became hydrophobic and adsorbed oil at high pH (pH = 13) or high temperature (T = 60 °C). The hydrophilic fabric could adsorb water nearly 6 times and the hydrophobic one could adsorb oil over 4 times its own weight. The water adsorption capacity of fabric at pH 6.5 was not as high as that at pH 1 since the former one was relative hydrophobic, suggesting that the adsorption process was driven by the surface energy of the fabric. By either increasing the pH or temperature, the system changed from hydrophilic to hydrophobic, and thereby, can be used for the separation of both water-rich and oil-rich mixtures.
The thermo-responsibility of pDMAEMA-grafted cotton can be tuned by altering the pH (Fig. 8b). When the fabric was pretreated with a solution of pH 1, the fabric remained hydrophilic with increasing temperature. No obvious transition was found in this case, while the fabric showed good temperature-sensibility at higher pH. The transition temperature of pDMAEMA-grafted fabric was about 45 °C at pH 6.5, which shifted to about 35 °C at pH 7. The reason may lie in the decreasing protonation degree and surface charge of the fabric resulting from increasing the pH. Less cationic groups lead to the decrease of electrostatic repulsion between pDMAEMA chains and their solubility in water, and thereby favor the collapse of chains, which could lower the transition temperature.28,30 When the pH = 13, the polymer is almost uncharged and the transition temperature shifts below 25 °C. Thus, we can say that the transition temperature of cotton-pDMAEMA fabrics is also strongly dependent on pH.
The LCST-type relationship between TT and pH was illustrated in Fig. 8c. By either rising the pH or temperature, pDMAEMA chains can change from soluble to insoluble state, and correspondingly, the system changed from “one phase” to “two phase”.31 The shadow region indicated the latter state, which was revealed by the high hydrophobicity of the material surface. The critical transition temperature of the fabric could be adjusted by controlling the protonation degree of the surface grafted pDMAEMA, which is related to the pH. The performance of the oil/water separation could be achieved by the cooperation effect of pH and temperature over a wide range. Compared to single-responsive material, the dual-responsive one is more intelligent and controllable for oil–water separation.
Self-cleaning performance
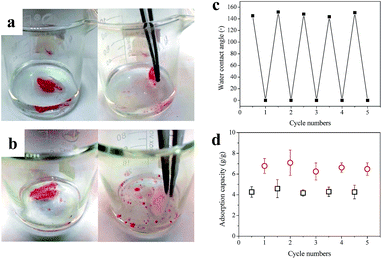
As is well-known, the oil-removing materials are apt to be fouled and even blocked up by oils.7,32 This made the materials difficult to re-use, and thereby limited their wider application. Also, it is unlikely for oil in the material to be collected and recycled. In this work, the dual-responsive fabric could not only separate oil/water mixture with a high efficiency, but also resisted oil fouling (Fig. 9). For raw cotton, the adsorption of oil was irreversible (Fig. 9a), while the pDMAEMA-grafted fabric was capable of desorbing the oil automatically in cold acidic solution after oil adsorption. As shown by Fig. 9b and the video S1,† oil droplets came out from the fabric and gathered on the surface, and immediately came off the fabric with a couple of wobbles. It was achieved by the reversible conversion of the pDMAEMA-grafted fabric between superoleophilicity and underwater superoleophobicity. At pH 1, pDMAEMA formed a highly-hydrated layer on the surface of the fibers, which could strongly repel oils. Thus, the oil droplets came off quickly from the fabric, making it be possible for the fabric to be re-used.In order to further study the reusability of the modified cotton fabric in oil/water separation application, the surface wettability and adsorption capacity was measured in each oil adsorption and releasing cycle (Fig. 9c). At high pH (half cycle), the fabric was hydrophobic with a water contact angle larger than 130°, and at low pH (complete cycle), the fabric was hydrophilic (CA = 0°). The hydrophobic fabric could easily recover its superhydrophilicity in aqueous media with a pH of 1, suggesting that the oil in the fabric was totally removed and had no impact on the chemical characteristics of the fabric. As discussed previously, the adsorption capacity strongly depends on the surface property of the textile-based matrix. Thus, as a result of the unchanged surface wettability, the pDMAEMA-grafted fabric took in a similar amount of oil or water after using many times. The oil adsorption capacity was kept above 4 times of its own weight. So, we can say, this reversible cycle could be repeated many times (>5 cycles) with an insignificant variation of the surface wettability and oil/water adsorption capacity, indicating the high performance of material recycling.
Conclusions
To produce a type of oil/water separation material with a high efficiency and durability, a dual-responsive polymer (pDMAEMA) was grafted onto cotton by ATRP. With a higher pDMAEMA grafting ratio, the fabric showed a higher temperature and pH responsive performance. In detail, the fabric switched from hydrophilic to hydrophobic with increasing temperature or pH. The transition temperature was about 45 °C and the transition pH was around 7. The fabric reached superhydrophilicity at low pH or temperature and high hydrophobicity at high temperature or pH. The fabric could adsorb oil nearly 4 times of its own weight and the oil could almost all be released in acid and cold water. The self-cleaning property is attributed to the reversible surface wettability, which allows for the recovery of the separation ability of the material. Our functionalized cotton fabrics are expected to become durable and effective materials for multicycle oil/water separation. Since cotton is a cheap and sustainable material, it shows promise for future underwater applications.Acknowledgements
This study is financially supported by the Natural Science Foundation of China (Grant No. 51303161) and China Postdoctoral Science Foundation (Grant No. 2014M561790).Notes and references
- G. Wang, Z. X. Zeng, X. D. Wu, T. H. Ren, J. Han and Q. J. Xue, Polym. Chem., 2014, 5, 5942–5948 RSC.
- Y. Z. Cao, X. Y. Zhang, L. Tao, K. Li, Z. X. Xue, L. Feng and Y. Wei, ACS Appl. Mater. Interfaces, 2013, 5, 4438–4442 CAS.
- M. Khosravi and S. Azizian, ACS Appl. Mater. Interfaces, 2015, 7, 25326–25333 CAS.
- Z. X. Xue, Y. Z. Cao, N. Liu, L. Feng and L. Jiang, J. Mater. Chem. A, 2014, 2, 2445–2460 CAS.
- F. Liu, M. L. Ma, D. L. Zang, Z. X. Gao and C. Y. Wang, Carbohydr. Polym., 2014, 103, 480–487 CrossRef CAS PubMed.
- M. Zhang, C. Y. Wang, S. L. Wang and J. Li, Carbohydr. Polym., 2013, 97, 59–64 CrossRef CAS PubMed.
- Z. X. Xue, S. T. Wang, L. Lin, L. Chen, M. J. Liu, L. Feng and L. Jiang, Adv. Mater., 2011, 23, 4270–4273 CrossRef CAS PubMed.
- F. Zhang, W. B. Zhang, Z. Shi, D. Wang, J. Jin and L. Jiang, Adv. Mater., 2013, 25, 4192–4198 CrossRef CAS PubMed.
- X. Y. Zhang, Z. Li, K. S. Liu and L. Jiang, Adv. Funct. Mater., 2013, 23, 2881–2886 CrossRef CAS.
- L. B. Zhang, Y. J. Zhong, D. Cha and P. Wang, Sci. Rep., 2013, 3 Search PubMed.
- Q. Wen, J. C. Di, L. Jiang, J. H. Yu and R. R. Xu, Chem. Sci., 2013, 4, 591–595 RSC.
- K. T. Huang, S. B. Yeh and C. J. Huang, ACS Appl. Mater. Interfaces, 2015, 7, 21021–21029 CAS.
- Y. Z. Cao, N. Liu, C. K. Fu, K. Li, L. Tao, L. Feng and Y. Wei, ACS Appl. Mater. Interfaces, 2014, 6, 2026–2030 CAS.
- Y. S. Ye, J. Huang and X. L. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 22128–22136 CAS.
- B. Wang and Z. G. Guo, Chem. Commun., 2013, 49, 9416–9418 RSC.
- D. L. Tian, X. F. Zhang, Y. Tian, Y. Wu, X. Wang, J. Zhai and L. Jiang, J. Mater. Chem., 2012, 22, 19652–19657 RSC.
- L. Yan, J. Li, W. J. Li, F. Zha, H. Feng and D. C. Hu, Mater. Lett., 2016, 163, 247–249 CrossRef CAS.
- Y. G. Jiang, P. B. Wan, M. Smet, Z. Q. Wang and X. Zhang, Adv. Mater., 2008, 20, 1972 CrossRef CAS.
- J. L. Zhang, X. Y. Lu, W. H. Huang and Y. C. Han, Macromol. Rapid Commun., 2005, 26, 477–480 CrossRef CAS.
- L. B. Zhang, Z. H. Zhang and P. Wang, NPG Asia Mater., 2012, 4, e8 CrossRef.
- W. Sun, S. X. Zhou, B. You and L. M. Wu, J. Mater. Chem. A, 2013, 1, 10646–10654 CAS.
- S. Dai, P. Ravi and K. C. Tam, Soft Matter, 2009, 5, 1987–1989 RSC.
- A. Tuteja, W. Choi, J. M. Mabry, G. H. McKinley and R. E. Cohen, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 18200–18205 CrossRef CAS PubMed.
- M. A. Shirgholami, M. S. Khalil-Abad, R. Khajavi and M. E. Yazdanshenas, J. Colloid Interface Sci., 2011, 359, 530–535 CrossRef CAS PubMed.
- J. D. Wu, Y. L. Jiang, D. J. Jiang, J. He, G. Q. Cai and J. P. Wang, Mater. Lett., 2015, 160, 384–387 CrossRef CAS.
- J. Q. Wang, D. Q. Song, S. S. Jia and Z. Q. Shao, React. Funct. Polym., 2014, 81, 8–13 CrossRef CAS.
- J. Niskanen, M. Karesoja, T. Rossi and H. Tenhu, Polym. Chem., 2011, 2, 2027–2036 RSC.
- X. Han, X. X. Zhang, H. F. Zhu, Q. Y. Yin, H. L. Liu and Y. Hu, Langmuir, 2013, 29, 1024–1034 CrossRef CAS PubMed.
- J. P. Zhang and S. Seeger, Adv. Funct. Mater., 2011, 21, 4699–4704 CrossRef CAS.
- D. Fournier, R. Hoogenboom, H. M. L. Thijs, R. M. Paulus and U. S. Schubert, Macromolecules, 2007, 40, 915–920 CrossRef CAS.
- F. A. Plamper, A. Schmalz, M. Ballauff and A. H. E. Muller, J. Am. Chem. Soc., 2007, 129, 14538 CrossRef CAS PubMed.
- A. K. Kota, G. Kwon, W. Choi, J. M. Mabry and A. Tuteja, Nat. Commun., 2012, 3, 1025 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Movies S1 is the oil releasing process of oil-captured fabric in acid aqueous solution. See DOI: 10.1039/c6ra02252a |
| This journal is © The Royal Society of Chemistry 2016 |