The effect of 2-mercaptobenzothiazole on laser-assisted electroless copper plating
Sisi Xianga,
Weiping Li*a,
Zhiyuan Qianb,
Liqun Zhua and
Huicong Liua
aKey Laboratory of Aerospace Materials and Performance (Ministry of Education), School of Materials Science and Engineering, Beihang University, 37# Xueyuan Road, Haidian District, Beijing 100191, China. E-mail: liweiping@buaa.edu.cn; Fax: +86 1082317133; Tel: +86 1082317113
bShanghai Key Laboratory of Spacecraft Mechanism, Shanghai 201108, China
First published on 13th April 2016
Abstract
The effect of 2-mercaptobenzothiazole (2-MBT) on laser-assisted electroless copper plating, using formaldehyde as a reducing agent as well as ethylenediaminetetraacetic acid (EDTA) and ethylenediaminetetrapropionic acid (EDTP) as complexing agents, has been investigated. The surface morphology, roughness, and crystallinity of copper coating depend on the 2-MBT concentration. The plating rate enhances significantly with the increase of 2-MBT contents. Accelerating mechanism of electroless copper plating has been studied by means of Tafel plots and linear sweep voltammetry. It is found that the appropriate amount of 2-MBT can improve the quality of copper coatings and accelerate the electroless copper plating process due to the promotion of the controlling step, as 2-MBT additions can form new electrostatic forces resulting in the decrease of absorption of [CH2(OH)O−]ad or Had and then make the controlling step occur more easily.
1. Introduction
Laser-assisted electroless copper plating, also known as autocatalytic plating, has been widely used in the microelectronics industries for the conductive treatment of internal antenna and printed circuit boards.1,2 Compared to the traditional electroless copper plating requiring complicated activation by PdCl2, laser-assisted electroless copper plating has significantly simplified the pretreatment and lowered the cost,3 thus optimization of subsequent process is necessary for wider application. Electroless copper plating bath generally contains cupric salt, reducing agent, complexing agent and additives like stabilizers or accelerators. Formaldehyde has been traditionally used as the reducing agent in the electroless copper plating because of high plating rate and low cost.4,5 However, the copper coating will be loose and the coating appearance becomes dark or brown with the increase of plating rate.6,7 Mechanical and physical properties depend mainly on quality of copper coatings.8 Therefore, high quality of copper coatings is required with a high plating rate at the same time. Additives, such as thiourea, 2,2-dypiridil, 2-MBT, monoamine, cyanide and certain metal ions, are proved effectively on improving quality of coatings used in the electroless copper plating bath.9–11 While addition of some additives have other harmful side-effect, such as 2,2-dypiridil addition in bath would decrease plating rate much,12 some amine additions can accelerate the plating process sharply but a loose and dark coating will be prepared.13Among these additives, 2-MBT is especially attractive because while improving coating quality, it accelerates the electroless copper plating process rather than decelerating it.14,15 To date, the understanding of the plating process with 2-MBT is very limited. Previous works mainly focused on optimizing process while effects of 2-MBT additions on the electroless copper plating process are still remain not fully understood.6,16–19 2-MBT has been used as stabilizer to improve the bath stability and coating quality.20–23 Paunovic et al.9 had studied some stabilizers working in electroless copper plating and finally proved effect of additives have been correlated to their electronic structure of molecules. Besides, Zheng18 and Hung10 had finished an investigation about the effects of plating parameters such as pH values and plating temperature, amount of stabilizers or other factors on the plating process based on results of Bindra,24 who had used electrochemical measurements to analyze the process of electroless copper plating using formaldehyde as reducing agent, including the anode oxidation of HCHO and cathode reduction of Cu2+. Since 2-MBT is used as stabilizer so that there's little research related to the acceleration process with 2-MBT additions.
In this study, we mainly focused on analyzing how 2-MBT worked in laser-assisted electroless copper plating bath, composing of formaldehyde as the reducing agent and EDTA and EDTP as complexing agents. Effects of 2-MBT on coating quality and rate in the plating bath were investigated. Accelerating mechanism was mainly analyzed by electrochemical measurements.
2. Experimental details
2.1 Fabrication of copper coatings on PET substrates
The fabrication process of electroless copper plating on PET substrates, used as internal antenna, requires pretreatment including precleaning, laser direct structuring and plating procedures.2.2 Characterization techniques
CHI-660A electrochemical workstation was used for electrochemical measurements. Tafel plots were obtained in electroless copper bath at a scan rate of 1 mV s−1. The linear sweep voltammetry (LSV) was carried out at 60 °C at a scan rate of 5 mV s−1. A three-electrode cell, comprising of a pure copper working electrode with 1 cm2 surface area, a Pt counter electrode, and an Hg/Hg2Cl2 reference electrode (SCE), was used to perform the measurement. For comparison, bath for anode polarization measurement was the same as basic plating bath but without Cu2+.Plating rate was evaluated by gravimetrical testing. Glossiness was measured by portable vancometer (XGP 20–60–85°). Surface and microscopic morphology were obtained by video microscope (KH-7700) and scanning electron microscope (SEM, JOEL-7500). The crystal structure of the copper deposits was investigated by X-ray diffraction (XRD, Rigaku 2000). Diffraction data were acquired over scattering angle 2θ from 40° to 80° at the rate of 6° min−1 with step size of 0.02°. The effective grain size of copper coatings is estimated by the use of Scherrer's equation:25
3. Result and discussion
3.1 Effect on coating quality
Color and glossiness of copper coatings prepared by various concentration of 2-MBT is shown in Table 1. With the addition of 2-MBT, the color of coatings turned darker while surface glossiness increased, suggesting smoother surface obtained. The glossiness had a disproportionally significant increase when the concentration of 2-MBT was increased to 3 mg L−1. However, the coating could not completely cover the surface at this concentration, suggesting that amount of 2-MBT behaved as catalytic poisons, making the reduction of copper ions harder or even impossible, therefore, amount of 2-MBT should be limited lower than a certain value to maintain its effectiveness.Fig. 1 shows the surface morphology of copper coatings prepared with various concentrations of 2-MBT in the plating bath. Each three-dimension graph, as composed of multiple colors, presents the roughness degree of copper coatings. According to the Color Scale automatic generated from KH-7700 3D viewer at the top right corner of each graph, height values decreased by 63.6% from 0.805 μm to 0.293 μm with increasing the 2-MBT concentration from 0 to 2 mg L−1, shown in Fig. 1(a) and (d). It suggested that smoother coatings were obtained, in agreement with the results of glossiness shown in Table 1.
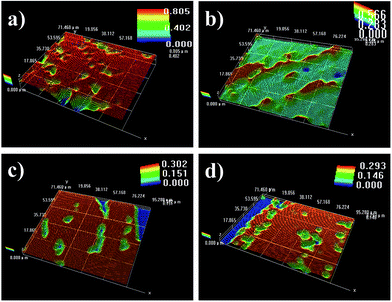 | ||
| Fig. 1 Effects of 2-MBT at different concentrations on surface morphology. (a) 0 mg L−1; (b) 0.8 mg L−1; (c) 1.6 mg L−1; (d) 2 mg L−1. | ||
Effect of 2-MBT at different concentrations on micro-morphology is illustrated in Fig. 2. The copper coatings became more compact and higher crystal refinement was achieved with the addition of 2-MBT. 2-MBT additions will help form chelates with the Cu+ and reduce the activity of the Cu0 nuclei, leading to a poor stability of plating bath. Therefore, addition of 2-MBT into plating bath will help to form a more homogeneous and compact copper coating.
 | ||
| Fig. 2 SEM images of copper coatings prepared with addition of various concentrations of 2-MBT. (a) 0 mg L−1; (b) 0.8 mg L−1; (c) 1.6 mg L−1; (d) 2 mg L−1. | ||
The insets at the top right corner are higher magnification pictures of each coating. It can be obviously observed from the insets of Fig. 2(b) and (c) that preferred growth of copper coating occurs with the contents of 2-MBT lower than 2 mg L−1. However, when increasing 2-MBT to 2 mg L−1 in the plating bath, preferred growth direction became not as significant and topography became smoother, as shown in Fig. 2(d).
The XRD patterns of the copper coatings obtained at different 2-MBT concentrations are shown in Fig. 3. The peaks appearing at 43.2°, 50.3° and 74.1° represented (111), (200) and (220) planes of crystalline copper, respectively. The copper oxide phase was not detected in the coatings. The effective grain size (D) of copper coatings listed in Table 2 is estimated from the (111) planes in the diffraction peaks by the use of Scherrer's equation.
| Concentration of 2-MBT (mg L−1) | Grain size (nm) | Intensity (%) | ||
|---|---|---|---|---|
| (111) | (200) | (220) | ||
| 0 | 109.6 | 100 | 29.8 | 19.8 |
| 0.8 | 47.4 | 100 | 18.5 | 15.9 |
| 1.6 | 35.5 | 100 | 16.2 | 16.4 |
Table 2 shows the grain size and intensity of (111), (200) and (220) crystal planes. Grain size of copper coatings decreased by 77.6% with the addition of 2-MBT up to 2 mg L−1 (111) plane orientation was intensified with the addition of 2-MBT up to 1.6 mg L−1, but when 2-MBT reached 2 mg L−1 in the plating bath, (111) preferred growth was not as obvious, perfectly corresponding to the results of SEM images, which may be caused by the over addition of 2-MBT leading to the excess coverage on the copper surface.
3.2 Effect on the plating rate and mechanism discussion
Fig. 4 shows the plating rate of electroless copper as a function of 2-MBT concentration in the plating bath. The plating rate was not affected significantly by 2-MBT addition up to about 1.4 mg L−1, and then increased from 7.1 μm h−1 to a maximum value of 8.2 μm h−1 with the increase of 2-MBT concentration to 1.6 mg L−1. When continuing to increase 2-MBT concentration, the plating rate decreased sharply. Furthermore, complete copper coatings couldn't be prepared when adding more than 2 mg L−1 2-MBT into basic bath, as shown in Table 1. Compared to the basic bath without 2-MBT, the peak rate of electroless copper plating increased by 15.5%.Fig. 5 shows the absorption of 2-MBT on active copper surface. There are one benzene ring and one heterocyclic ring of 2-MBT forming π bonding structure together. Copper has a very high affinity towards sulphur,23 thus 2-MBT has a strong coordination with the active copper surface as illuminated in Fig. 5. The initial increase of plating rate by the addition of 2-MBT may be explained with the presence of delocalized π electron bond in the structure. This phenomenon is attributed to great adsorption ability at low concentrations of 2-MBT on the active copper surface, which in turn facilitates the copper reduction with sufficient supplement of π electron and accelerates the process. While on the other hand, the decrease of plating rate may be related to excess coverage on the copper surface. The effect of delocalized π electron and surface absorption work simultaneously at the active copper surface, resulting in a peak plating rate at about 1.4 mg L−1 addition of 2-MBT, in agreement with researches of Nuzzi16 and Hung.10
Based on results above, we have a further investigation of 2-MBT with presence in electroless copper plating process. Fig. 6 is Tafel plots measured in the basic bath with different concentrations of 2-MBT, and open circuit potential (E0) and exchange current density (i0) were obtained, as listed in Table 3. The reversible reaction occurs at E0 (about −0.7 V) is listed as follow:26
| Cu2+ + 2HCHO + 4OH− ↔ Cu + 2HCOO− + 2H2O + H2↑ |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i0 (A cm−2) from Tafel curves
i0 (A cm−2) from Tafel curves
| Concentration of 2-MBT (mg L−1) | E0 vs. SCE (V) | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i0 (A cm−2) i0 (A cm−2) |
|---|---|---|
| 0 | −0.712 | −4.460 |
| 0.8 | −0.692 | −2.779 |
| 2.5 | −0.705 | −6.893 |
E0 shifted positively with the increase of 2-MBT contents, suggesting the reduction process occurred more easily with addition of 2-MBT. When adding 0.8 mg L−1 2-MBT into the plating bath, i0 significantly increased, which means the rate of reverse and forward reaction both increased. However, when using 2.5 mg L−1 2-MBT, i0 instead dropped to lower than that of the basic plating bath, indicating that this reversible reaction was restrained. Therefore, both taking the effects of E0 and i0 on copper deposition into account, the reduction reaction occurred more easily with addition of a certain amount of 2-MBT. Nevertheless, copper deposition was depressed with excess addition of 2-MBT due to the significant decrease of i0, causing by the excessive absorption thus the decrease of copper surface area for reaction, though positive shift of E0 was beneficial to the deposition process.
Effect of 2-MBT on formaldehyde oxidation polarization curves is expressed in Fig. 7. 0 mg L−1, 0.5 mg L−1, 1.0 mg L−1, 1.5 mg L−1 and 3 mg L−1 2-MBT were added into the plating bath respectively. Results from Fig. 7 points out that three different oxidation peaks occur at the anode potential of −0.60 V (Peak 1), −0.45 V (Peak 2) and −0.2 V (Peak 3), respectively, in the oxidation process of electroless copper plating. It can be seen that Peak 1 decreased at first, whereas Peak 2 increased simultaneously with the addition of less than 3 mg L−1 2-MBT into the bath. An opposite phenomenon, namely the increase of Peak 1 and the decrease of Peak 2, occurred with excess 2-MBT (3 mg L−1). Curves from Fig. 7 are compatible with the following sequence of steps:24
| CH2(OH)O− ↔ [CH2(OH)O−]ad | (1) |
| [CH2(OH)O−]ad + OH− → HCOO− + H2O + Had + e− | (2) |
| Had + Had ↔ H2↑ | (3) |
| Had + OH− ↔ H2O + e− | (4) |
 | ||
| Fig. 7 HCHO oxidation polarization curves at different concentrations of 2-MBT. (a) 0 mg L−1; (b) 0.5 mg L−1; (c) 1.0 mg L−1; (d) 1.5 mg L−1; (e) 3 mg L−1. | ||
Paunovic9 had proved that addition of some stabilizers with π bonding structure could form new electrostatic forces resulting in the decrease of absorption of product and intermediate of formaldehyde in the overall reaction of electroless copper plating, thus 2-MBT may also be explained in terms of the same argument based on the results. With a certain amount addition of 2-MBT, the decrease of [CH2(OH)O−]ad or Had made the reaction (3) occur harder, corresponding to the reaction of Peak 1. At the potential of −0.60 V, there was an irreversible reaction of adsorbed HCHO with hydrogen evolution occurring as follow:
| 2HCHO + 4OH− → 2HCOO− + 2H2O + H2↑ + 2e− | (5) |
On the other hand, plating bath with high pH could provide enough OH− for the process of reaction (4), corresponding to the reaction of Peak 2. Reaction of adsorbed HCHO without hydrogen evolution occurs at the potential of −0.45 V as follow:
| HCHO + 3OH− → HCOO− + 2H2O + 2e− | (6) |
In addition a reaction about oxidation of copper occurs at the potential of −0.2 V, as follow:
| Cu → Cu2+ + 2e− | (7) |
And these three reactions in the process of formaldehyde oxidation well agree with the results of Zheng.18 Comparing with the change law of plating rate, the variation of Peak 2 well attaches to that of the whole plating rate shown in Fig. 4, suggesting that reaction of Peak 2 is the controlling step during formaldehyde oxidation process. Therefore, it can be inferred that a certain amount of 2-MBT accelerates the plating process by promoting the controlling step, which can form new electrostatic forces resulting in the decrease of absorption of [CH2(OH)O−]ad or Had and then make the reaction of controlling step occur more easily. While with excess 2-MBT additions, absorption dominates the whole process, making Cu2+ reduction occur harder or even impossible, neither does the HCHO oxidation.
4. Conclusion
2-MBT has successfully improved both the coating quality and plating rate in laser-assisted electroless copper plating process. Uniform and dense surface of copper coating was obtained. The plating rate reached a maximum value of 8.2 μm h−1 with basic bath containing 1.6 mg L−1 2-MBT, increasing by 15.5% in comparison to free 2-MBT bath.Furthermore, the effect of delocalized π electron and surface absorption work simultaneously at the active copper surface, resulting in a peak plating rate. During electroless copper plating process, the amount of 2-MBT would significantly influence E0 and i0. With a certain amount of 2-MBT added in the bath, E0 shifted positively and i0 increased, both making the copper deposition more easily. 2-MBT additions will help form new electrostatic forces resulting in the decrease of absorption of HCHO intermediate making the reaction of controlling step occur more easily and finally accelerates the whole electroless copper plating process.
Acknowledgements
The authors are very grateful to the support from the Shanghai Key Laboratory of Spacecraft Mechanism.References
- H. Narcus, Met. Finish., 1947, 45, 964–967 Search PubMed.
- C. A. Deckert, Plat. Surf. Finish., 1995, 82, 48–55 CAS.
- A. Radke, T. Gissibl, T. Klotzbücher, P. V. Braun and H. Giessen, Adv. Mater., 2011, 23, 3018–3021 CrossRef CAS PubMed.
- J. Dumesic, J. A. Koutsky and T. W. Chapman, J. Electrochem. Soc., 1974, 121, 1405–1412 CrossRef CAS.
- X. Gan, K. Zhou, W. Hu and D. Zhang, Surf. Coat. Technol., 2012, 206, 3405–3409 CrossRef CAS.
- M. Matsuoka, J. Murai and C. Iwakura, J. Electrochem. Soc., 1992, 139, 2466–2470 CrossRef CAS.
- M. Oita, M. Matsuoka and C. Iwakura, Electrochim. Acta, 1997, 42, 1435–1440 CrossRef CAS.
- S. Nakahara and Y. Okinaka, Annu. Rev. Mater. Sci., 1991, 21, 93–129 CrossRef CAS.
- M. Paunovic and R. Arndt, J. Electrochem. Soc., 1983, 130, 794–799 CrossRef CAS.
- A. Hung, J. Electrochem. Soc., 1985, 132, 1047–1049 CrossRef CAS.
- L. L. Duda, Plat. Surf. Finish., 1998, 85, 60–62 CAS.
- J. Li, H. Hayden and P. A. Kohl, Electrochim. Acta, 2004, 49, 1789–1795 CrossRef CAS.
- K. Kondo, J. Ishikawa, O. Takenaka, T. Matsubara and M. Irie, J. Electrochem. Soc., 1990, 137, 1859–1860 CrossRef CAS.
- L. Zan, Z. Liu, Z. Yang and Z. Wang, Electrochem. Solid-State Lett., 2011, 14, D107–D109 CrossRef CAS.
- N. Sato and M. Suzuki, J. Electrochem. Soc., 1988, 135, 1645–1650 CrossRef CAS.
- F. J. Nuzzi, Plat. Surf. Finish., 1983, 70, 51–54 CAS.
- K. Mishra and R. Paramguru, J. Electrochem. Soc., 1996, 143, 510–516 CrossRef CAS.
- Y.-j. Zheng, F.-x. Xiao, W.-h. Zou and Y. Wang, J. Cent. South Univ. Technol., 2008, 669–673 CrossRef CAS.
- J. Van Den Meerakker, J. Appl. Electrochem., 1981, 11, 387–393 CrossRef CAS.
- T. L. Aycock, N. C. Huie and G. Krauss, Metall. Trans., 1974, 5, 1215–1223 CrossRef CAS.
- L. N. Schoenberg, J. Electrochem. Soc., 1972, 119, 1491–1493 CrossRef CAS.
- R. L. Coombes, Plating, 1970, 57, 675 Search PubMed.
- M. Ohsawa and W. Süetaka, Corros. Sci., 1979, 19, 709–722 CrossRef CAS.
- P. Bindra and J. Roldan, J. Electrochem. Soc., 1985, 132, 2581–2589 CrossRef CAS.
- B. D. Cullity, in Elements of X-ray Diffraction, Addison-Wesley, London, 2nd edn, 1978 Search PubMed.
- J. A. Dean, in Lange's handbook of chemistry, 1985 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |









