Biphenyl derivatives containing trimethylsilyl benzyl ether or oxime groups as probes for NO2 detection†
L. Alberto Juárezabd,
Ana M. Costero*abd,
Margarita Parraabd,
Salvador Gilabd,
Javier Ródenasab,
Félix Sancenónacd and
Ramón Martínez-Máñez*acd
aInstituto Interuniversitario de Reconocimiento Molecular y Desarrollo Tecnológico (IDM), Unidad Mixta Universidad Politécnica de Valencia-Universidad de Valencia, Spain
bDepartamento de Química Orgánica. Universidad de Valencia, Doctor Moliner 50, Burjassot, 46100, Valencia, Spain. E-mail: ana.costero@uv.es
cDepartamento de Química, Universidad Politécnica de Valencia, Camino de Vera s/n, 46022, Valencia, Spain. E-mail: rmaez@qim.upv.es
dCIBER de Bioingeniería, Biomateriales y Nanomedicina (CIBER-BBN), Spain
First published on 26th April 2016
Abstract
Four probes based in the use of a biphenyl moiety and functionalized with trimethylsilyl benzyl ether (P1 and P3) and oxime (P2 and P4) groups have been prepared and tested as optical probes for the detection of NO2. Reaction of NO2 with acetonitrile solutions of P2–P4 resulted in the formation of aldehydes 7 and 8 with a concomitant redshift of the absorption bands. Probe P2 displayed a bathochromic shift of 45 nm upon reaction with NO2 and was able to detect this poisonous gas at concentrations as low as 0.02 ppm. P2 was highly selective against NO2 and other gases (i.e. NO, CO2, H2S, SO2) and vapours of organic solvents (i.e. acetone, hexane, chloroform, acetonitrile or toluene) had no effect in the optical properties of the probe.
Introduction
Nitrogen oxides (NOx) are formed in large quantities from fuel combustion in cars, trucks and power plants;1 they are a major problem in urban areas and are linked to many respiratory diseases.2 From a chemical point of view NOx mainly refers to the sum of nitric oxide (NO) and nitrogen dioxide (NO2), although other nitrogen species can also be included, such as nitrous and nitric acids. Together with the adverse effects of direct exposure to NOx on human health, it is also remarkable its contribution to ground level ozone and fine particle pollution. Hence, strict regulations regarding levels of nitrogen oxides are currently applied by governments and the monitorization of NOx levels based in reliable analytical methods is of great interest.3Among NOx species, NO2 causes a range of harmful effects on lungs such as increased inflammation of the airways, worsened cough and wheezing, reduced lung function, increased asthma attacks and increased susceptibility to respiratory infection.4 All these problems are more important for children and older adults.5 Due to the ubiquitous presence of NO2, the development of selective and sensitive sensing methods for its detection is a hot area of research.6 No standards have been agreed upon for nitrogen oxides in indoor air, moreover ASHRAE and the US EPA National Ambient Air Quality Standards list 0.053 ppm as the average 24 hour limit for NO2 in outdoor air.7 However, NO2 levels in certain cities and at certain hours can reach even higher values (near 100 ppm).
Laser-based photoacoustic spectroscopy,8 surface acoustic wave (SAW),9 transition metal oxide devices,10 carbon quantum dot-functionalized aerogels,11 or ozone treated graphene12 are some reported analytical techniques used to detect/monitor NO2 levels. However, some of these methods show certain limitations such as lack of specificity, limited selectivity, operational complexity, non-portability, difficulties in real-time monitoring and false positive readings. As an alternative to these instrumental procedures, the development of molecular chemosensors, constructed under the chemodosimeter paradigm, has been gaining interest in recent years. However, the number of publications related with optical probes for NO2 detection is still relatively scarce.13
Bearing in mind our interest in the development of chemical sensors for gas detection,14 we report herein the synthesis and sensing behavior towards NO2 of four new chemodosimeters based on the biphenyl chromophore. Among the different organic reactions involving NO2 we decided to explore the utility of the generation of aromatic aldehydes from trimethylsilyl benzyl ethers15 and oximes16 in order to prepare suitable chemodosimeters for the detection of this poisonous and pollutant gas. These are reactions with quantitative yields, working at room temperature and at ambient pressure. The synthesized biphenyl probes (vide infra) possess electron donor groups (such as methoxide electronically connected with trimethylsilyl) or oxime (weak electron acceptor) moieties. The underlying idea is that, upon NO2 reaction, the formed aldehyde (an electron acceptor moiety) would change the electronic properties of the chemodosimeter with subsequent shifts of the absorption bands (see Scheme 1).
Results and discussion
Probes P1–P4 were prepared following the synthetic pathway depicted in Scheme 2. Pd(0) catalyzed cross-coupling reaction of the appropriate boronic acids (1 and 2) with 4-bromohydroxymethylbenzene (3) or 4-bromobenzaldehyde (4) yielded the corresponding biphenyl derivatives bearing hydroxyl (5 and 6) or aldehyde (7 and 8) moieties.17 Transformation of the hydroxyl group into the corresponding trimethylsilyl ether (probes P1 and P2) was carried out using hexametildisilazane (HMDS) in dry CH2Cl2,18 whereas the probes containing oximes (P3 and P4) were obtained with hydroxylamine hydrochloride in H2O/MeOH mixed with sodium carbonate.19 All compounds were characterized by 1H NMR, 13C NMR and MS (see ESI†). Acetonitrile solutions of the four probes (1.0 × 10−4 M) showed intense absorption bands in the 260–300 nm region with ε values ranging from 6000 to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1.
000 M−1 cm−1.
In a first step, the absorption changes of probes P1–P4 (1.0 × 10−4 M in acetonitrile) after bubbling 1 ppm of NO2 during 5 min (obtained from a commercial cylinder), was tested.
Acetonitrile solutions of P1 showed an absorption band centered at 277 nm that was redshifted to 285 nm upon bubbling air containing 1 ppm of NO2 (see Fig. 1). Interestingly, the same absorption band centered at 285 nm was observed upon bubbling NO2 into acetonitrile solutions of probe P3 (see also Fig. 1). The new absorption band, formed upon NO2 bubbling, was ascribed to the formation of aldehyde 7 as a consequence of the oxidative deprotection of the trimethylsilyl ether moiety in P1 and of the rupture of the oxime group in P3. In fact both, the UV and 1H NMR spectra of aldehyde 7, were fully coincident with that obtained after treatment of probes P1 and P3 with NO2. Optically, the transformation of an electron donor (trimethylsilyl ether in P1) or a weak electron acceptor (oxime in P3) group into an aldehyde (with a marked ability to attract electronic density) yielding 7, resulted in a bathochromic shift of the absorption band of P1 and P3. A similar redshifts of the absorption bands of acetonitrile solutions of P2 and P4 was observed upon bubbling NO2 (1 ppm in air) (see Fig. 1). These changes were ascribed to the reaction of NO2 with the probes that yielded in both cases aldehyde 8. Also in this case it was found that the UV and 1H NMR spectra of aldehyde 8, were fully coincident with that obtained after treatment of probes P2 and P4 with NO2. The best sensing performance, in terms of a larger shift of the absorption band, was obtained for probe P2, for which a redshift of 45 nm (from 264 to 309 nm) was observed upon bubbling 1 ppm of NO2. For this reason, further detailed studies with P2 were carried out in order to assess the sensitivity and selectivity of this probe toward NO2.
The limit of detection (LOD) using P2 for NO2 was determined by UV measurements by bubbling increasing amounts of NO2 into an acetonitrile solution of the probe. As shown in Fig. 2, the absorbance at 309 nm was gradually enhanced when the concentration of NO2 increases. From the titration profile a LOD as low as 0.02 ppm was calculated.
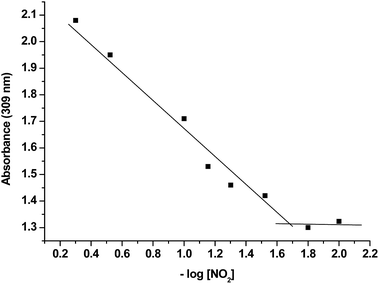 | ||
| Fig. 2 Absorbance at 309 nm measured after bubbling increasing quantities of NO2 into acetonitrile solutions of probe P2 (1.0 × 10−4 M). | ||
In a second step, the selectivity of P2 was assessed. This is an important issue in the design of probes for pollutant gases in order to overcome potential interferents or false-positive readings produced by other species. Taking this into account, the potential reactivity of probe P2 with other hazardous gases (i.e. NO, CO2, H2S, SO2) or organic vapors (i.e. acetone, hexane, chloroform, acetonitrile, toluene) was tested by bubbling the selected species into acetonitrile solution of P2 (1.0 × 10−4 M). None of the gases tested, at concentrations up to 100 ppm, induced changes in the UV spectra of probe P2 indicating a high selective reaction of P2 with NO2 (see Fig. 3).
 | ||
| Fig. 3 Absorbance at 309 nm of probe P2 alone and in the presence of selected potential interferents (all compounds at a concentration of 100 ppm). | ||
Competitive studies were also carried out. Thus, Fig. 3 shows that the absorption of the band at 309 nm of probe P2 in the presence of a complex gas mixture (NO2 + NO + CO2 + H2S + SO2) was the same than that observed when NO2 was used alone. This result demonstrated a high selective response of probe P2 toward NO2 and suggested its possible use for the detection/monitoring of this poisonous gas.
Conclusions
Four new biphenyl-based probes P1–P4 have been synthesized and used for the selective recognition of NO2. For all four probes a bathochromic shift of the absorption bands was observed in the presence of the NO2 and ascribed to the generation of an aromatic aldehyde upon reaction of NO2 with trimethylsilyl benzyl ether or oxime groups contained in P1–P2 and P3–P4, respectively. Among the prepared chemosensors, P2 showed the higher shift of the absorption band and for this probe a LOD as low as 0.02 ppm was determined for NO2 detection. Moreover, the response of P2 was highly selective and no reaction was found in the presence of NO, CO2, H2S, SO2 or organic vapors of acetone, hexane, chloroform, acetonitrile or toluene. We believe that these, or similar probes based in the same chemical reaction, can display a large potential as optical probes for the selective detection of NO2.Experimental section
General remarks
Dichloromethane and acetonitrile were distilled from P2O5 under Ar prior to use. Silica gel 60 F254 (Merck) plates were used for TLC. Colum chromatography was performed on silica gel. 1H and 13C NMR spectra were determined on a Bruker AV 300 spectrometer. Chemical shifts are reported in parts per million (ppm), calibrated to the solvent peak set. High-resolution mass spectra were recorded in the positive ion mode with a VG-AutoSpec mass spectrometer. Absorption and fluorescence spectra were recorded using a Shimadzu UV-2600 spectrophotometer.Synthesis of biphenyl alcohols 5 and 6
In a typical run, the corresponding boronic acid (1 for the synthesis of 5; 2 for the preparation of 6) (2 mmol) was added, to a solution of 3 (1 mmol) in DMF (20 mL) in the presence of sodium carbonate (6 mmol). Afterward, the flask was evacuated and refilled with argon. Then, tetrakis(triphenylphosphine)palladium(0) was added and the crude obtained heated at 100 °C for 30 minutes with vigorous stirring. The resultant mixture was diluted with H2O (10 mL) and Et2O (10 mL), followed by extraction twice with Et2O. The ethereal extract was collected and the solvent evaporated under vacuum. The final product was isolated by column chromatography on silica, with hexane/ethyl acetate (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as eluent, yielding a colourless solid (65%).
2) as eluent, yielding a colourless solid (65%).
5
(65%), colourless solid. 1H NMR (300 MHz, DMSO) δ (ppm): 7.68–7.60 (m, 4H), 7.50–7.32 (m, 5H), 5.21 (t, J = 5.7 Hz, 1H), 4.54 (d, J = 5.7 Hz, 2H). 13C NMR (75 MHz, DMSO) δ (ppm): 142.2, 140.5, 138.9, 129.3, 127.6, 127.4, 126.9, 126.7, 62.9.6
(67%), colourless solid. 1H NMR (300 MHz, DMSO) δ (ppm): 7.50–7.43 (m, 4H), 7.37 (d, J = 8.4 Hz, 2H), 6.91 (d, J = 8.7 Hz, 2H), 5.20 (t, J = 5.7 Hz, 1H), 4.53 (d, J = 5.7 Hz, 2H), 3.79 (s, 3H). 13C NMR (75 MHz, DMSO) δ (ppm): 159.1, 132.9, 132.8, 132.7, 127.9, 127.4, 126.2, 114.7, 63.0, 55.6.Synthesis of probes P1 and P2
HDMS (40 mmol) was added to the corresponding alcohol (20 mmol) in dry dichloromethane (20 mL). The mixture was stirred at room temperature for 22 h (full conversion) under argon atmosphere. The solvent was evaporated and the crude was purified by column chromatography on silica using hexane/ethyl acetate (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as eluent, to give the probes P1 (90%) or P2 (93%) as white solids.
2) as eluent, to give the probes P1 (90%) or P2 (93%) as white solids.
P1
(90%), white solid, mp 158–160 °C. 1H NMR (300 MHz, DMSO) δ (ppm): 7.50 (m, 4H), 7.27 (m, 5H), 4.56 (s, 2H), 0.00 (s, 9H). 13C NMR (75 MHz, DMSO) δ (ppm): 140.5, 140.3, 139.2, 129.2, 127.6, 127.3, 126.9, 63.8, −0.1. UV-Vis (acetonitrile) λmax = 277 nm (ε = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 M−1 cm−1).
300 M−1 cm−1).
P2
(93%), white solid, mp 170–173 °C. 1H NMR (300 MHz, DMSO) δ (ppm): 7.45 (m, 4H), 7.22 (d, J = 8.4 Hz, 2H), 6.88 (d, J = 8.5 Hz, 2H), 4.55 (s, 2H), 3.66 (s, 3H), 0.00 (s, 9H). 13C NMR (75 MHz, DMSO) δ (ppm): 139.6, 138.8, 132.6, 129.2, 127.9, 127.3, 126.2, 114.6, 63.8, 0.1. UV-Vis (acetonitrile) λmax = 264 nm (ε = 8000 M−1 cm−1).Synthesis of 7 and 8
4 (1.5 mmol) and 1 or 2 for 7 and 8 respectively (3 mmol) were dissolved in DMF (20 mL). Afterward, sodium carbonate (9 mmol) was added to this solution. The crude reaction was stirred under inert atmosphere for 30 min. Then, a catalytic amount of tetrakis(triphenylphosphine)palladium(0) was added and the reaction was stirred at 100 °C for 10 minutes. After this time water (10 mL) was added and the mixture was extracted with ethyl acetate (2 × 20 mL). The organic phase was washed with brine (2 × 20 mL), dried with MgSO4 and evaporated to give the product.7 was purified by silica column chromatography with hexane/ethyl acetate (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give a white crystalline solid (82%). 1H NMR (300 MHz, DMSO-d6) δ 10.06 (s, 1H), 8.01 (m, 2H), 7.92 (d, J = 8.3 Hz, 2H), 7.78 (m, 2H), 7.50 (m, 3H). 13C NMR (75 MHz, DMSO-d6) δ 193.20, 146.34, 139.27, 135.56, 130.62, 129.60, 129.06, 127.84, 127.60. HRMS (EI): m/z calc. for C13H10O 182.07 [M + 1]+ found: 183.0797 UV-Vis (acetonitrile) λmax = 289 nm (ε = 9500 M−1 cm−1).
1) to give a white crystalline solid (82%). 1H NMR (300 MHz, DMSO-d6) δ 10.06 (s, 1H), 8.01 (m, 2H), 7.92 (d, J = 8.3 Hz, 2H), 7.78 (m, 2H), 7.50 (m, 3H). 13C NMR (75 MHz, DMSO-d6) δ 193.20, 146.34, 139.27, 135.56, 130.62, 129.60, 129.06, 127.84, 127.60. HRMS (EI): m/z calc. for C13H10O 182.07 [M + 1]+ found: 183.0797 UV-Vis (acetonitrile) λmax = 289 nm (ε = 9500 M−1 cm−1).
8 was purified by silica column chromatography with hexane/ethyl acetate (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to give a white crystalline solid (75%). 1H NMR (300 MHz, DMSO-d6) δ 10.03 (s, 1H), 8.01–7.93 (m, 2H), 7.91–7.85 (m, 2H), 7.74 (d, J = 9.0 Hz, 2H), 7.08 (d, J = 8.9 Hz, 2H), 3.82 (s, 3H). 13C NMR (75 MHz, DMSO) δ (ppm): 192.9, 160.2, 145.9, 134.8, 131.3, 130.5, 128.7, 127.0, 114.9, 55.6. HRMS (EI): m/z calc. for C14H12O2 212.08 [M + 1]+ found: 213.0901. UV-Vis (acetonitrile) λmax = 309 nm (ε = 12
2) to give a white crystalline solid (75%). 1H NMR (300 MHz, DMSO-d6) δ 10.03 (s, 1H), 8.01–7.93 (m, 2H), 7.91–7.85 (m, 2H), 7.74 (d, J = 9.0 Hz, 2H), 7.08 (d, J = 8.9 Hz, 2H), 3.82 (s, 3H). 13C NMR (75 MHz, DMSO) δ (ppm): 192.9, 160.2, 145.9, 134.8, 131.3, 130.5, 128.7, 127.0, 114.9, 55.6. HRMS (EI): m/z calc. for C14H12O2 212.08 [M + 1]+ found: 213.0901. UV-Vis (acetonitrile) λmax = 309 nm (ε = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 M−1 cm−1).
700 M−1 cm−1).
Synthesis of probes P3 and P4
The corresponding aldehyde (7 and 8 for P3 and P4 respectively, 2 mmol) and hydroxylamine hydrochloride (2.2 mmol) were dissolved in methanol–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 40 mL). A previously prepared solution of sodium carbonate (2 mmol) in water was slowly added and the reaction was stirred for 3 h at room temperature. Then, methanol was evaporated and the aqueous phase was extracted with ether (4 × 40 mL). The organic phase was washed with brine (1 × 30 mL) and dried with MgSO4. After evaporation of the solvent probes P3 (99%) and P4 (77%) were isolated as white solids.
1, 40 mL). A previously prepared solution of sodium carbonate (2 mmol) in water was slowly added and the reaction was stirred for 3 h at room temperature. Then, methanol was evaporated and the aqueous phase was extracted with ether (4 × 40 mL). The organic phase was washed with brine (1 × 30 mL) and dried with MgSO4. After evaporation of the solvent probes P3 (99%) and P4 (77%) were isolated as white solids.
P3
(99%), white crystalline solid, mp 142–145 °C. 1H NMR (300 MHz, DMSO-d6) δ 11.28 (s, 1H), 8.19 (s, 1H), 7.70 (m, 6H), 7.48 (m, 2H) 7.40 (m, 1H). 13C NMR (75 MHz, DMSO) δ (ppm): 148.1, 141.2, 139.8, 132.6, 129.3, 128.1, 127.3, 127.3, 126.9. HRMS (EI): m/z calc. for C13H11NO 197.08 [M + 1]+ found: 198.0913. UV-Vis (acetonitrile) λmax = 282 nm (ε = 5800 M−1 cm−1).P4
(77%), white crystalline solid, mp 165–167 °C. 1H NMR (300 MHz, chloroform-d) δ 8.09 (s, 1H), 7.50 (m, 5H), 6.92 (d, J = 8.9 Hz, 2H), 5.23 (s, 1H), 3.79 (s, 3H). 13C NMR (75 MHz, DMSO) δ (ppm): 159.5, 148.2, 140.9, 134.8, 130.5, 128.7, 128.1, 127.0, 114.9, 55.6. HRMS (EI): m/z calc. for C14H13NO2 227.09 [M + 1]+ found: 228.1019. UV-Vis (acetonitrile) λmax = 300 nm (ε = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 M−1 cm−1).
800 M−1 cm−1).
Limits of detection measurements
Increasing quantities of NO2 gas from commercially available NO2 cylinder were bubbled for 5 min through a solution of P2 in acetonitrile. The UV spectra were recorded in 1 cm path length cells at 25 °C. Representation of the wavelength (nm) vs. concentration of NO2 allowed the limit of detection to be calculated by using the eqn (1)| LOD = 3sb/m | (1) |
Acknowledgements
We thank the Spanish Government and FEDER founds (MAT2012-38429-C04) and Generalitat Valenciana (PROMETEOII/2014/047) for support. SCSIE (Universidad de Valencia) is gratefully acknowledged for all the equipment employed.References
- K. Skalska, J. S. Miller and S. Ledakowicz, Sci. Total Environ., 2010, 408, 3976–3989 CrossRef CAS PubMed.
- S. C. Barman, N. Kumar, R. Singh, G. C. Kisku, A. H. Khan, M. M. Kidwai, R. C. Murthy, M. P. S. Negi, P. Pandey, A. K. Verma, G. Jain and S. K. Bhargava, J. Environ. Biol., 2010, 31, 913–920 CAS.
- Analytical Techniques for Atmospheric Measurement, ed. D. Heard, John Wiley & Sons, 2008, p. 331 Search PubMed.
- (a) J. L. Devalia, A. M. Campbell, R. J. Sapsford, C. Rusznak, D. Quint, P. Godard, J. Bousquet and R. J. Davies, Am. J. Respir. Cell Mol. Biol., 1993, 9, 271–278 CrossRef CAS PubMed; (b) S. M. Horvath, Bull. N. Y. Acad. Med., 1980, 56, 835–846 CAS.
- (a) W. S. Tunnicliffe, P. S. Burge and J. G. Ayres, Lancet, 1994, 344, 1733–1736 CrossRef CAS PubMed; (b) M. Shima and M. Adachi, Int. J. Mol. Epidemiol. Genet., 2000, 29, 862–870 CrossRef CAS.
- (a) H. Dehghani, M. Bezhgi, R. Malekzadeh, E. Imani, S. Pasban-Noghabi, G. Javadi, R. Faraji, M. Negahdary and R. Aghebati-Maleki, Int. J. Electrochem. Sci., 2014, 9, 1454–1467 Search PubMed; (b) M. Zhao, T. Zhang, J. Wang and Z. Yuan, J. Network, 2013, 8, 405–412 Search PubMed; (c) J. Z. Ou, W. Ge, B. Carey, T. Daeneke, A. Rotbart, W. Shan, Y. Wang, Z. Fu, A. F. Chrimes, W. Wlodarski, S. P. Russo, Y. X. Li and K. Kalantar-Zadeh, ACS Nano, 2015, 9, 10313–10323 CrossRef CAS PubMed.
- U.S. Environmental Protection Agency (EPA), Non-cancer Health Effects (RELs), Office of Environmental Health Hazard Assessment (OEHHA), California, DC, USA, 1999 Search PubMed.
- A. Mukherjee, M. Prasanna, M. Lane, R. Go, I. Dunayevskiy, A. Tsekoun, C. Kumar and N. Patel, Appl. Opt., 2008, 47, 4884–4887 CrossRef CAS PubMed.
- A. Venema, E. Nieuwkoop, M. J. Vellekoop, M. S. Nieuwenhuizen and A. W. Barendsz, Sens. Actuators, 1986, 10, 47–64 CrossRef CAS.
- (a) W. K. Nomani, D. Kersey, J. James, D. Diwan, T. Vogt, R. A. Webb and G. Koley, Sens. Actuators, B, 2011, 160, 251–259 CrossRef; (b) D. Zhang, Z. Liu, C. Li, T. Tang, X. Liu, S. Han, B. Lei and C. Zhou, Nano Lett., 2004, 4, 1919–1924 CrossRef CAS; (c) S.-W. Choi, A. Katoch, G. J. Sun, P. Wu and S. S. Kim, J. Mater. Chem. C, 2013, 1, 2834–2841 RSC; (d) X. Liang, S. Yang, J. Li, H. Zhang, Q. Diao, W. Zhao and G. Lu, Sens. Actuators, B, 2011, 158, 1–8 CrossRef CAS.
- R. Wang, G. Li, Y. Dong, Y. Chi and G. Chen, Anal. Chem., 2013, 85, 8065–8069 CrossRef CAS PubMed.
- M. G. Chung, D. H. Kim, H. M. Lee, T. Kim, J. H. Choi, D. K. Seo, J.-B. Yoo, S.-H. Hong, T. J. Kang and Y. H. Kim, Sens. Actuators, B, 2012, 166–167, 172–176 CrossRef CAS.
- (a) L. A. Juárez, A. M. Costero, M. Parra, S. Gil, F. Sancenón and R. Martínez-Máñez, Chem. Commun., 2015, 51, 1725–1727 RSC; (b) J. Mokhari, M. R. Naimi-Jamal and H. Hamzehal, 11th International Electronic Conference on Synthetic Organic Chemistry (ECSOC-11), November 2007, pp. 1–30 Search PubMed.
- (a) A. Martí, A. M. Costero, P. Gaviña and M. Parra, Chem. Commun., 2015, 51, 3077–3079 RSC; (b) L. A. Juárez, A. Barba-Bon, A. M. Costero, R. Martínez-Máñez, F. Sancenón, M. Parra, P. Gaviña, M. C. Terencio and M. J. Alcaraz, Chem.–Eur. J., 2015, 21, 15450 CrossRef.
- S. Ohira, E. Wanigasekara, R. D. Rudkevich and P. K. Dasgupta, Talanta, 2009, 79, 14–20 Search PubMed.
- M. Javaheri, M. R. Naimi-Jamal, M. G. Dekamin and G. Kaupp, Phosphorus, Sulfur Silicon Relat. Elem., 2012, 187, 142–148 CrossRef CAS.
- C. M. Nunes and A. L. Monteiro, J. Braz. Chem. Soc., 2007, 18, 1443–1447 CrossRef CAS.
- J. Marjan, Tetrahedron, 2012, 68, 3861–3867 CrossRef.
- S. Castellano, D. Kuck, M. Viviano, J. Yoo, F. López-Vallejo, P. Conti, L. Tamborini, A. Pinto, J. L. Medina-Franco and Y. G. Sbardella, J. Med. Chem., 2011, 54, 7663–7677 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra02222g |
| This journal is © The Royal Society of Chemistry 2016 |



