Fabrication of poly(cyclotriphosphazene-co-4,4′-sulfonyldiphenol) nanotubes decorated with Ag–Au bimetallic nanoparticles with enhanced catalytic activity for the reduction of 4-nitrophenol
Abstract
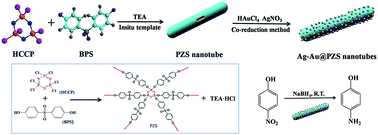
Ag–Au bimetallic nanoparticles (NPs) are deposited on poly(cyclotriphosphazene-co-4,4′-sulfonyldiphenol) (PZS) nanotubes by a facile and effective co-reduction method, wherein the PZS nanotubes with abundant hydroxyl groups have been prepared via an in situ template approach. Upon varying the feeding amounts of the Ag and Au precursors, the bimetallic compositions of the PZS nanotubes can be readily tuned resulting in a series of bimetallic catalysts with different Ag to Au molar ratios, thus leading to the tunable catalytic properties. Characterization results show that the Ag–Au bimetallic nanoparticles with smaller size and good dispersibility are well anchored onto the surface of the PZS nanotubes. Furthermore, the reduction of 4-nitrophenol (4-NP) into 4-aminophenol (4-AP) by NaBH4 is applied as a model reaction to study the effect of different Ag-to-Au molar ratios on the catalytic capabilities of the resulting composites. It is found that the catalytic capability is remarkably enhanced when the Au content is increased. The maximum activity parameter value reaches 92.2 s−1 g−1, which is far higher than that of PZS nanotubes decorated with either Ag or Au nanoparticles alone.

 Please wait while we load your content...
Please wait while we load your content...