Direct energy harvesting from starch by hybrid enzymatic and non-enzymatic cascade bioanode
Zonghua Wang*a,
Lin Xia*a,
Jianfei Xiaa,
Zhendong Anb and
Shida Gonga
aCollaborative Innovation Center for Marine Biomass Fiber, Materials and Textiles of Shandong Province, Shandong Sino-Japanese Center for Collaborative Research of Carbon Nanomaterials, College of Chemistry and Chemical Engineering, Laboratory of Fiber Materials and Modern Textiles, The Growing Base for State Key Laboratory, Qingdao University, Qingdao, Shandong 266071, China. E-mail: wangzonghua@qdu.edu.cn; 45489957@qq.com
bSunrui Marine Environment Engineering Co., Ltd., Qingdao, Shandong 266101, China
First published on 2nd March 2016
Abstract
A hybrid anode integrating enzymatic hydrolysis of starch by glucoamylase and non-enzymatic oxidation of glucose by gold nanoparticles is presented to achieve an efficient cascade energy conversion from starch. The as-prepared biofuel cell shows a maximum power output of 91.4 μW cm−2. This enzymatic/non-enzymatic hybrid strategy can be employed to develop other polysaccharide or oligosaccharide fuel cells in which glucose oxidation is involved.
Introduction
Biofuel cells (BFCs) are electrochemical devices that use enzymes or microorganisms as biocatalysts to convert chemical energy stored in organic matter to electricity via bio-catalytic reactions.1 Due to the increasing demand for energy and the depletion of fossil fuels, BFCs, as a new source of sustainable and renewable energy, have attracted great research interest recent years.2 Although enzymes demonstrate superior catalytic efficiency in mild conditions including ambient temperatures and neutral pH, limited oxidation degree of fuel sources caused by high substrate specificity and short lifetime due to the instability of enzymes are still two major issues existing in the present development of biofuel cells.3,4 To improve the energy density and expand the potential fuel source for BFCs application, multi-enzyme immobilization strategies4–6 were widely reported, however the complicated electron transfer and diffusion pathway as well as the enzymatic activity compromised by system cross-talking make the multi-enzyme system still challengeable. Recently, the hybrid enzyme and non-enzyme cascade system for the production of high value chemicals7 and complete oxidation of biological fuels have also been successfully demonstrated.8Among biological origin fuels, glucose is mostly selected for BFCs research because of its promising application for implantable power devices which utilize blood sugar as fuel source. However, in other field of application, considering the energy density and the cost of fuels, polysaccharide or oligosaccharides may be more desirable candidates. Starch is the most common carbohydrate found in many plants, it consists of a large number of glucose units joined by glycosidic bonds. Compared to glucose and other carbohydrate, it is cheaper and higher in energy density. Glucoamylase (GA) is a starch hydrolyzing enzyme which can efficiently catalyze the hydrolysis of α-(1,4) glycosidic bonds at the non-reducing end of starch polymer to release free glucose.9
Recent years, intensive research have been done on non-enzymatic oxidation of glucose aimed at reliable non-enzymatic glucose sensors. Among various nanomaterials attempted, nanostructured gold appears to be one of the most promising candidate because of its faster glucose oxidation reaction rate in neutral and alkaline conditions and better biocompatibility.10–12 Recent studies on glucose oxidation by gold nanoparticles (AuNPs) also reveals that the use of direct electrochemical approaches11,13 or seed mediated growth techniques allow the fabrication of pure AuNPs without the catalytically hindering effect of the stabilizing molecules which commonly used in chemical synthesis approach. Therefore, with appropriate integration techniques, the pure AuNPs can be a desirable candidate for highly efficient cascade catalytic reaction component.
In this work, a hybrid anode integrating enzymatic hydrolysis of starch and non-enzymatic oxidation of glucose is presented for starch fuel cell application. The direct deposition of AuNPs and separate modification of GA via cross-linker on carbon nanofibers surface enables the effective hydrolysis of starch to glucose and subsequent highly efficient glucose oxidation by pure AuNPs and therefore the direct energy harvesting from starch.
Materials and methods
Chemicals
Glucoamylase (GA, α-1,4-glucan-gluco-hydrolase, EC.3.2.1.3) was purchased from Toyobo. Ltd. 1-Pyrenebutanoic acid succinimidyl ester, tetra-chloroauric acid (HAuCl4·3H2O) were purchased from Sigma Aldrich. Dimethylformamide (DMF) was purchased from Alfa Aesar. Carbon nanofibers (CNFs) were purchased from XFNANO, China. Wet proofed carbon cloth and platinum catalysts were purchased from FuelCell Store, USA.Fabrication of AuNPs decorated CNFs modified glassy carbon electrode (AuNPs/CF/GCE)
The glassy carbon electrode (GCE, 3 mm in diameter) was polished carefully with 0.3 and 0.05 μm alumina slurry, sonicated in anhydrous ethanol and deionized water, respectively, and dried with nitrogen blow before use. Then 8 μl of CNFs (3 mg ml−1 dispersed in DMF solution) was casted on GCE and dried in 37 °C flow air oven for 2 hours. Afterward, gold nanoparticles were deposited by reduction of 0.2 mM HAuCl4 solution at a constant potential of −0.2 V for 10–15 min (adjusted from a previously reported method14).Immobilization of GA on AuNPs/CNF/GCE
GA were linked to the CNFs using a previously reported heterobifunctional cross-linker, 1-pyrenebutanoic acid succinimidyl ester (PBSE), which provides covalent binding with amino groups of protein lysine residues through the formation of amide bonds and interacts with CNFs via π–π stacking of the polyaromatic pyrenyl moieties, the procedure was followed as reported.15,16 After cross-linking, the electrode was washed with DDW and then subjected to a polarization process in a phosphate buffer solution (PBS) (pH = 7.4), at a potential of −0.1 V maintained for 10–15 min, in order to remove the other physically-adsorbed residues on the AuNPs surface.For performance comparison, AuNPs/CNFs/GCE anode with physically adsorbed GA was prepared by dipping the AuNPs/CNFs/GCE anode 2 mg ml−1 of GA solution (pH 5.5, 50 mM acetate buffer) for 4 hours and then washed 3 times with acetate buffer prior to use.
Measurements and characterization
All the electrochemical experiments were performed by VMP3 Potentiostat/Galvanostat (EG&G, Princeton Applied Research) with in a conventional three-electrode based electrochemical cell at room temperature (ca. 23 °C), the hybrid bioanode was used as a working electrode, a graphite rod as an auxiliary electrode and an Ag/AgCl electrode as the reference electrode. Linear Sweep Voltammetry (LSV) was used to study the electro-catalytic performance of the as prepared hybrid anode, scan range is from −0.6 V to 0.6 V, scan rate is 50 mV s−1.The morphologies of AuNPs/CNFs were investigated by Hitachi S-4800 field emission scanning electron microscope (FE-SEM).
Fuel cell construction and performance characterization
The assembled electrodes served as the anode in a single compartment semi-biofuel cell (10 ml); consisting of 0.1 M PBS buffer (pH 7.4), starch (1.5% wt). The air cathode was fabricated as following: the wet-proofed carbon cloth was coated with platinum (0.5 mg cm−2) with a surface area of 6 cm2 (2 × 3 cm) was used as cathode (Fuel Cell Earth, USA). The electrochemically simulated cathode was potentiostatically controlled, using a three-electrode configuration: graphite plate (1 cm2) as a working electrode, platinum wire as a counter electrode and Ag/AgCl as a reference electrode. The cathode was biased to a potential of +500 mV against Ag/AgCl. The voltage generated from the biofuel cell was measured by a hand held multimeter (DM-97, Sinometer, China). Various external resistances were applied between the anode and cathode by a resistance decade box (RBOX 408, Lutron Electronic Enterprise, Taipei, Taiwan). The generated voltage at each resistance was measured after reaching equilibrium. Measurements were carried out at ambient temperature. For the continuous measurement carried out for the operational stability test, the output voltage was recorded by VMP3 Potentiostat/Galvanostat (EG&G, Princeton Applied Research) with an external resistor of 30 kΩ.Results and discussion
To achieve an efficient glucose oxidation at a lower potential and neutral pH, a higher degree of surface active sites on the AuNPs surface is indispensable.17 So as the first step, the direct electrochemical deposition method was employed to obtain pure AuNPs without any stabilizing molecules on CNFs. The SEM image of pure AuNPs decorated CNFs was shown in Fig. 1A, the average diameter of the AuNPs was about 11 nm (measured and calculated from SEM image) and uniformly distributed on the CNFs surface. Besides direct electrochemical deposition of AuNPs on CNFs, a heterobifunctional cross-linker, PBSE was employed to link the GA by covalent binding with amino groups of protein lysine residues through the formation of amide bonds and attach on CNFs via π–π stacking of the polyaromatic pyrenyl moieties, as shown in Scheme 1.The electro-catalytic performance of the as prepared hybrid anode toward starch was studied by Linear Sweep Voltammetry (LSV). As shown in Fig. 1B, with the presence of 1% (wt) starch in PBS solution, the evolution of anodic electro-catalytic current resulting from the oxidation of glucose was clearly visible (curve b), the glucose oxidation starts at ca. −0.4 V and a large oxidation peak observed at −0.06 V is related to the adsorption of glucose on the AuNPs surface and subsequent formation of gluconolactone involving a two-electron transfer reaction.18 Another small oxidation peak followed at approximately 0.27 V in the anodic direction could be assigned to the continuous oxidation of gluconolactone.13 As a comparison shown in Fig. 1C, LSVs of the AuNPs/CNFs electrode with physically adsorbed GA show a very limited glucose oxidation activity (curve b), there is no peaked observed around −0.1 V, which may indicate that the catalytically hindering effect caused by GA adsorption on AuNPs significantly impairs the earlier onset potential of glucose oxidation. From Fig. 1D, no electrochemical catalytic current was observed when using the AuNPs/CNFs electrode without GA as anode, only a slight current drop was found due to the solution resistance increase upon the addition of starch.
Afterward, BFCs were assembled based on GA/AuNPs/CNFs hybrid anode. For the hybrid anode performance evaluation, both electrochemically simulated cathode and air cathode were employed for fuel cell performance test. To remove the performance variation influenced by the practical biocathode, firstly, electrochemically simulated cathode was employed during the fuel cell test to evaluate different anode performance.
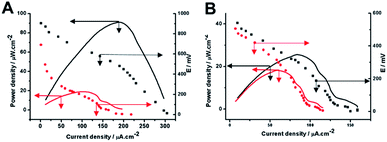
The cathode was controlled by a potentiostat similarly to the way it was described by Schröder et al.,19 and was biased continuously at +500 mV vs. Ag/AgCl electrode. The fuel at the anode was starch, and the fuel cell was of one compartment configuration and exposed to the ambient atmosphere in neutral pH. The performance of the as assembled BFCs is shown in Fig. 2. Fig. 2A shows the power output measured from different modified bioanodes based BFCs. The GA/AuNPs/CNFs anode based BFC shows a maximum power output of 91.4 μW cm−2, (black curve, solid line). In contrast, BFCs assembled by the AuNPs/CNFs electrode with physically adsorbed GA only displays a maximum power output of 18.9 μW cm−2, (red curve, solid line). The polarization curves of as-assembled BFCs were shown as in Fig. 2A (dashed plot), which suggest the GA/AuNPs/CNFs anode based BFC can generate a maximum current density (short circuit current density) of ca. 304 μA cm−2. When the GA/AuNPs/CNFs hybrid anode based BFCs were assembled with air cathodes, the fuel cell performance were shown in Fig. 2B. The GA/AuNPs/CNFs anode based BFC shows a maximum power output of 25.6 μW cm−2 (black curve, solid line), which is much lower than the power output obtained with an electrochemically simulated cathode. This results comparison indicates that the BFC performance was limited by the air cathode performance. In contrast, BFCs assembled by the AuNPs/CNFs electrode with physically adsorbed GA and air cathode displays a maximum power output of 17.6 μW cm−2, which is similar to the result obtained with an electrochemically simulated cathode, suggesting in this case the BFC performance was limited by the AuNPs/CNFs anode with physically adsorbed GA.
Finally, the operational stability and storage stability of the GA/AuNPs/CNFs hybrid anode was tested by the combination of electrochemically simulated cathode. Fig. 3A shows the current response of the as-assembled fuel cell continuously operating in 0.1 M PBS (pH 7.4) containing 1% starch under ambient condition at the load of 30 kΩ for 72 h. The solution was replenished with starch every 24 h. After 72 h, the cell can still retain 83% of its initial current density. The storage stability of the as-prepared hybrid anode was also tested by measuring its maximum power output every few day for 1 month. The anode was stored at 4 °C in PBS buffer when not used. As shown in Fig. 3B, the cell exhibits good stability (about 92% during a week span and about 82% for a month span). These results indicate that the hybrid of non-enzymatic and enzymatic anode gave rise to the enhanced long term stability.
 | ||
| Fig. 3 Operational stability (A) and storage stability (B) of GA/AuNPs/CNFs/GCE hybrid anode based BFC. | ||
Conclusions
In conclusion, efficient cascade energy conversion from starch was achieved by a hybrid anode integrating enzymatic hydrolysis of starch by GA and non-enzymatic oxidation of glucose by gold nanoparticles. The BFCs assembled from the as-prepared anode show a maximum power output of 91.4 μW cm−2 and excellent stability. Moreover, this enzymatic/non-enzymatic hybrid strategy can be employed to develop other polysaccharide or oligosaccharide fuel cells in which glucose oxidation was involved.Acknowledgements
We really appreciate the financial support from the National Natural Science Foundation of China (21203228, 21275082, 21405086 and 21475071), the Taishan Scholar Program of Shandong Province, the Natural Science Foundation of Shandong (ZR2014BQ001, and BS2014NJ023) and the Natural Science Foundation of Qingdao (13-1-4-128-jch and 13-1-4-202-jch).Notes and references
- R. A. Bullen, T. C. Arnot, J. B. Lakeman and F. C. Walsh, Biosens. Bioelectron., 2006, 21, 2015–2045 CrossRef CAS PubMed.
- J. A. Cracknell, K. A. Vincent and F. A. Armstrong, Chem. Rev., 2008, 108, 2439–2461 CrossRef CAS PubMed.
- D. Sokic-Lazic, R. L. Arechederra, B. L. Treu and S. D. Minteer, Electroanalysis, 2010, 22, 757–764 CrossRef CAS.
- D. P. Hickey, F. Giroud, D. W. Schmidtke, D. T. Glatzhofer and S. D. Minteer, ACS Catal., 2013, 3, 2729–2737 CrossRef CAS.
- Y. Handa, K. Yamagiwa, Y. Ikeda, Y. Yanagisawa, S. Watanabe, N. Yabuuchi and S. Komaba, ChemPhysChem, 2014, 15, 2145–2151 CrossRef CAS PubMed.
- Q. Lang, L. Yin, J. Shi, L. Li, L. Xia and A. Liu, Biosens. Bioelectron., 2014, 51, 158–163 CrossRef CAS PubMed.
- D. P. Hickey, M. S. McCammant, F. Giroud, M. S. Sigman and S. D. Minteer, J. Am. Chem. Soc., 2014, 136, 15917–15920 CrossRef CAS PubMed.
- H. H. P. Yiu and M. A. Keane, J. Chem. Technol. Biotechnol., 2012, 87, 583–594 CrossRef CAS.
- J. Marín-Navarro and J. Polaina, Appl. Microbiol. Biotechnol., 2011, 89, 1267–1273 CrossRef PubMed.
- M. Gougis, A. Tabet-Aoul, D. Ma and M. Mohamedi, Sens. Actuators, B, 2014, 193, 363–369 CrossRef CAS.
- M. Chu, Y. Zhang, L. Yang, Y. Tan, W. Deng, M. Ma, X. Su, Q. Xie and S. Yao, Energy Environ. Sci., 2013, 6, 3600–3604 CAS.
- Y.-G. Zhou, S. Yang, Q.-Y. Qian and X.-H. Xia, Electrochem. Commun., 2009, 11, 216–219 CrossRef CAS.
- H. Shu, L. Cao, G. Chang, H. He, Y. Zhang and Y. He, Electrochim. Acta, 2014, 132, 524–532 CrossRef CAS.
- J. Du, R. Yue, F. Ren, Z. Yao, F. Jiang, P. Yang and Y. Du, Gold Bull., 2013, 46, 137–144 CrossRef.
- R. J. Chen, Y. Zhang, D. Wang and H. Dai, J. Am. Chem. Soc., 2001, 123, 3838–3839 CrossRef CAS PubMed.
- L. Halámková, J. Halámek, V. Bocharova, A. Szczupak, L. Alfonta and E. Katz, J. Am. Chem. Soc., 2012, 134, 5040–5043 CrossRef PubMed.
- K. E. Toghill and R. G. Compton, Int. J. Electrochem. Sci., 2010, 5, 1246–1301 CAS.
- M. Tominaga, T. Shimazoe, M. Nagashima and I. Taniguchi, Electrochem. Commun., 2005, 7, 189–193 CrossRef CAS.
- U. Schröder, J. Nießen and F. Scholz, Angew. Chem., Int. Ed., 2003, 42, 2880–2883 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2016 |