Nanohydroxyapatite/cellulose nanocrystals/silk fibroin ternary scaffolds for rat calvarial defect regeneration†
Xiaoming Chenab,
Runmei Zhouc,
Bin Chend and
Jianting Chen*a
aDepartment of Orthopedic Spinal Surgery, Nanfang Hospital, Southern Medical University, Guangzhou 510515, China. E-mail: chenjt99@tom.com; Fax: +86-20-87640940; Tel: +86-20-87640940
bDepartment of Orthopedic Spinal Surgery, The 2nd Affiliated Hospital, University of South China, Hengyang 421001, China
cDepartment of Pharmacy, The 2nd Affiliated Hospital, University of South China, Hengyang 421001, China
dDepartment of Orthopedic Spinal Surgery, Chenzhou No. 1 People's Hospital, Chenzhou 423000, China
First published on 8th April 2016
Abstract
The purpose of this study was to design and characterise a novel biomimetic scaffold for the repair of critical size calvarial defects. In this study, we developed a new hydroxyapatite/cellulose nanocrystals/silk fibroin (HA/CNC/SF) scaffold by mixing a solution of SF with HA and CNC nanoparticles. The scaffold was fabricated by freeze-drying. The average pore size and porosity of the HA/CNC/SF scaffolds were 110 ± 7.3 μm and 90 ± 6.2%, respectively. The thermostability and mechanical properties of the scaffolds were significantly better than those of either SF, CNC/SF or HA/SF scaffolds. Additionally, the composite scaffold exhibited excellent biocompatibility and superior osteoconductivity. Hence, the efficacy of the HA/CNC/SF scaffold was evaluated for bone regeneration in the rat calvarial defect model. The results demonstrated that the rat calvarial defect healed with new-formed bone within 12 weeks of implantation and the degradation rate of the scaffold was a good match to the bone regeneration rate. Therefore, the HA/CNC/SF scaffold is considered to be a strong candidate for the repair of bone defects in bone tissue engineering.
1. Introduction
Bone lesions and defects, which often arise from trauma, skeletal diseases or tumour resections, can cause pain, significant limitation in quotidian activities and even lead to death.1,2 However, large bone defects are difficult to heal by bone regeneration and self-healing. The traditional treatment of bone defects frequently uses autologous bone grafting and alloplastic implants.3 In general, the current standard treatment of bone defects uses autografts, which are often not precluded by risks of disease transfer and histo-incompatibilities, but by a lack of sufficient autograft tissue.4,5 However, the use of alloplastic implants is associated with risks of pathogen transmission, infection transmission and even implant failure.6 Tissue engineering has emerged as a potential alternative strategy to regenerate bone.7 Biomaterial scaffolds play central roles in regenerative medicine and tissue engineering, and promote tissue regeneration by directing cellular behaviours and functions.8 Accordingly, the essential properties required for an ideal scaffold in bone tissue engineering include good osteoconductivity, or even osteoinductivity, mineralisation and suitable mechanical properties, high porosity with interconnected structures and a composition of biocompatible and degradable biomaterials.9–11 Hence, it is desirable to devise a biomimetic bone tissue engineering scaffold for the repair of bone defects.Among various biomaterials, hydroxyapatite (HA), the main inorganic component of bone, has attracted much attention for large bone defect regeneration because of its good osteoinductivity, osteoconductivity and osteointegration.12–14 Cellulose nanocrystals (CNC), obtained by sulphuric acid hydrolysis of native cellulose, has been studied as a carrier of demineralised bone matrix with encouraging results in vivo.15 However, the low compressive strength of HA and CNC has limited their applications to non/low-load bearing bone repairs. The traditional way to improve the compressive strength of HA and CNC for use in functional scaffolds is to provide a uniform distribution of HA or CNC particles in the polymer matrix. Chen et al. prepared a bioactive biogas/chitosan/CNC composite hydrogel for bone regeneration.16 The composite hydrogel had a haemostatic effect and its biodegradation led to the functional reconstruction of bone defects. Silk fibroin (SF), a natural macromolecular polymer derived from the silkworm Bombyx mori, has superior mechanical strength, excellent biocompatibility, long-term biodegradability and is easily processed.17,18 Various studies have investigated using HA/SF scaffolds as bone defect repair materials; the results suggest that HA/SF scaffolds could be an excellent choice for bone regeneration.19
The HA, CNC, SF and the composites with one or two of them have been widely in previous studies. But, there are fewer researchers used the composites with all of them for the repair of bone defects in our knowledge. Ren et al. developed a nanoparticulate mineralized collagen glycosaminoglycan scaffold that induces healing of critical-sized rabbit cranial defects without addition of expanded stem cells or exogenous growth factors, the results demonstrated that the nanoparticulate mineralized collagen glycosaminoglycan scaffolds useful for bone regeneration.20 To develop a novel easy to use scaffold with enhanced bone repair properties, HA/CNC/SF scaffold was fabricated using lyophilisation. The morphology and properties of the HA/CNC/SF scaffold were investigated and the cellular compatibility of this scaffold was evaluated. The rat calvarial defect model was used to evaluate the efficacy of the HA/CNC/SF scaffold for bone regeneration in vivo.
2. Materials and methods
2.1. Materials
B. mori silkworm cocoons and MC3T3-E1 Subclone 14 were kindly donated by Sijia Min from Zhejiang University. A cell counting kit (CCK-8, Dojindo, Japan) was purchased from Sigma (Sigma, St. Louis, MO, USA). The CNC (Fig. S1†) were produced by sulphuric acid hydrolysis of microcrystalline cellulose and obtained from the Department of Biomedical Engineering, Jinan University. All other reagents were of analytical reagent quality and used without further processing.2.2. Preparation of HA
Nano-sized HA particles were prepared as previously described with minor modifications.21 Briefly, 100 mL of 0.3 M ammonium hydrogen phosphate solution was added dropwise into 100 mL of 0.5 M calcium chloride solution at 60 °C under magnetic stirring for 2 h. Meanwhile, the reaction mixture pH was kept at 10 by adding ammonium hydroxide. After aging for 24 h at room temperature, the precipitates were collected by centrifugation and washed five times with deionised water. Finally, the resulting precipitates were collected and then freeze-dried for 24 h to yield nano-sized HA particles.2.3. Preparation of the SF solution
The SF solution was prepared according to the previously described method.22 Briefly, the cocoons of the silkworm B. mori were cut into small pieces and then boiled in 0.5% (w/v) Na2CO3 aqueous solution for 1 h and washed with distilled water. This boiling and washing process was repeated twice to remove sericin from the silk fibres. The extracted silk fibroin were dried in an oven at 50 °C for 24 h and then dissolved in 9.3 M LiBr aqueous solution (mass to liquid ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) for 5 h at 60 °C to generate the SF solution. The solution was dialysed (Mw = 10 kDa) against deionised water for 3 days by changing the water daily. The solution was collected by centrifugation (5000 rpm, 10 min, 25 °C) and finally stored at 4 °C. The final concentration of the SF solution was ca. 4% (w/v), which was determined gravimetrically after freeze-drying for 24 h to remove any remaining solvent.
10) for 5 h at 60 °C to generate the SF solution. The solution was dialysed (Mw = 10 kDa) against deionised water for 3 days by changing the water daily. The solution was collected by centrifugation (5000 rpm, 10 min, 25 °C) and finally stored at 4 °C. The final concentration of the SF solution was ca. 4% (w/v), which was determined gravimetrically after freeze-drying for 24 h to remove any remaining solvent.
2.4. Preparation of SF-based scaffolds
The HA/CNC/SF scaffold was produced by mixing 1 mL of the 4% (w/v) SF solution, 10 mg of CNC and 10 mg of HA and then pouring the solution into a 48-well plate and freeze-drying for 24 h. The HA/SF scaffold was fabricated by mixing 1 mL of the 4% (w/v) SF solution and 10 mg of HA and then pouring the mixture into a 48-well plate and freeze-drying for 24 h. The CNC/SF scaffold was fabricated by mixing 1 mL of the 4% (w/v) SF solution and 10 mg of CNC and then pouring the mixture into a 48-well plate and freeze-drying for 24 h. The pure SF scaffold was also prepared. All SF-based scaffolds were immersed in 90% (v/v) aqueous methanol solution for 30 min to induce a structural transition that generated the water-insoluble SF scaffolds.232.5. Morphology and characterisation
Micrographs of the nano-sized HA particles and CNC were characterised with a Philips CM30 electron microscope operating at 100 kV. The morphologies of HA and the different SF-based scaffolds were also examined by scanning electron microscopy (SEM; LEO1530 VP; Philips, Amsterdam, The Netherlands). The pore sizes of the scaffolds were evaluated by measuring 25 random pores in the SEM images of the same sample using ImageJ freeware (NIH, Bethesda, MD, USA). The porosity of the SF scaffolds was measured according to a previously published method.24The phase and crystallinity of the nano-sized HA particles were determined by X-ray powder diffraction (XRD) using Cu Kα radiation (D8 Advance diffractometer; Bruker, Billerica, MA, USA). The infrared spectra of HA, CNC and the SF-based scaffolds were obtained using a Fourier transform infrared (FTIR) spectrometer (Vertex 70; Bruker). The spectra were recorded as KBr dispersions within the spectral region of 400–4000 cm−1 at a resolution of 4 cm−1 and 20 scans per sample. The thermal stability of HA, CNC and the SF-based scaffolds were studied using a thermogravimetric analyser (TGA; TG 209 F3; Netzsch, Exton, PA, USA). Samples (5–10 mg) were heated from room temperature to 800 °C at a constant heating rate of 10 °C min−1 in a nitrogen atmosphere. Compressive load and extension of the SF-based scaffolds were investigated using the Bose ElectroForce-3220 instrument (TA Instruments, Eden Prairie, MN, USA) equipped with a 225 N load cell at room temperature. The crosshead speed was 1 mm min−1 and at least three replicates of each type of specimen were evaluated.
2.6. In vitro cell proliferation assay of scaffolds
The MC3T3-E1 cells were cultured in α-MEM medium supplemented with 10% FBS and 100 U mL−1 antibiotics (penicillin–streptomycin) at 37 °C in a 5% CO2 incubator. The media were changed twice weekly.The cell proliferation was measured using the CCK-8 assay kit as previously reported.25 Briefly, the SF-based scaffolds were immersed in 75% ethanol for 2 h and then repeatedly rinsed with phosphate buffer solution (PBS) to remove residual ethanol. Subsequently, the cells were seeded with the SF-based scaffold and then the cells were cultured at 37 °C in a 5% CO2 incubator for 1, 3, 5 and 7 days. CCK-8 solution (10 μL) was subsequently added to each well and incubated for 3 h in an incubator. The absorbance of each well was measured using a microplate absorbance reader (Model 680; Bio-Rad, Hercules, CA, USA) at 450 nm. All experiments were performed in triplicate.
2.7. Alkaline phosphatase (ALP) activity
The ALP activity was analysed according to a previously reported method.8 The scaffolds were removed on days 3, 7 and 14 and washed with PBS to remove the residual serum. The cells on the scaffolds were dissolved in 500 μL of Millipore water containing 0.02% Triton X-100. Subsequently, the supernatant was collected by centrifugation at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and 4 °C for 15 min and then assayed according to the manufacturer's instructions. The absorbance at 405 nm was measured. Three replicate samples were used for the assay.
000 rpm and 4 °C for 15 min and then assayed according to the manufacturer's instructions. The absorbance at 405 nm was measured. Three replicate samples were used for the assay.
2.8. In vivo implantation and tissue collection
All procedures and handling of the animals were carried out in accordance with the guidelines of Jinan University and the US National Institutes of Health, and approved by the Animal Ethics Committee of Jinan University. Forty-eight male SD rats (200–250 g) were used in the study and randomly divided into four groups: HA/CNC/SF, HA/SF, CNC/SF and SF groups. Food and water were supplied ad libitum.For the calvarial defect model, the surgical procedures were performed as previously described.26 Briefly, the rats were anaesthetised by intraperitoneal injection of pentobarbital sodium (Sigma-Aldrich, St. Louis, MO, USA) at 30 mg kg−1. Subsequently, a 20 mm linear incision was shaved on the dorsal part of the cranium and the periosteum was removed by blunt dissection. A 6 mm full-thickness bone defect was created in the parietal bone with a slow-speed dental drill (Fig. S2B†). Physiological saline (0.9%) was irrigated for cooling and the dura mater was kept intact during the procedure. After the defects were implanted with the HA/CNC/SF, HA/SF, CNC/SF or SF scaffolds (Fig. S2C†), the skin was closed by suturing (Fig. S2D†). Samples were harvested at 4, 8 and 12 weeks postoperative.
2.9. Micro-computed tomography (CT)
The rats were anaesthetised at 4, 8 or 12 weeks after implantation. The calvarial defects were assessed using a micro-CT imaging system (Aloka LaTheta LCT-200; Hitachi Aloka Medical, Wallingford, CT, USA) and the volume of the regenerated bone within the defects was calculated using its auxiliary software. The three-dimensional representation of the defect site was reconstructed using the Mimics 15.0 software (Materialise, Inc., Leuven, Belgium).2.10. Histology evaluation
The rats were sacrificed at 4, 8 or 12 weeks after implantation. The skull was removed and fixed in 10% buffered formalin (pH 7.4) for 48 h at 37 °C. For further analysis, samples were decalcified in 10% EDTA for 4 weeks at room temperature, dehydrated using an ascending alcohol gradient, embedded in paraffin for staining with hematoxylin and eosin (H&E) and Safranin–Fast Green stain, and then observed with a light microscope (Axio Scope A1 FL; Carl Zeiss, Wetzlar, Germany).2.11. Statistical analysis
The experimental results are presented as means ± SD. All studies were performed in triplicate (n ≥ 3). All results were analysed using one-way ANOVA with replication and a combination of fixed and random variables. A p-value of less than 0.05 (p < 0.5) was considered significant.3. Results
3.1. Characteristics of HA
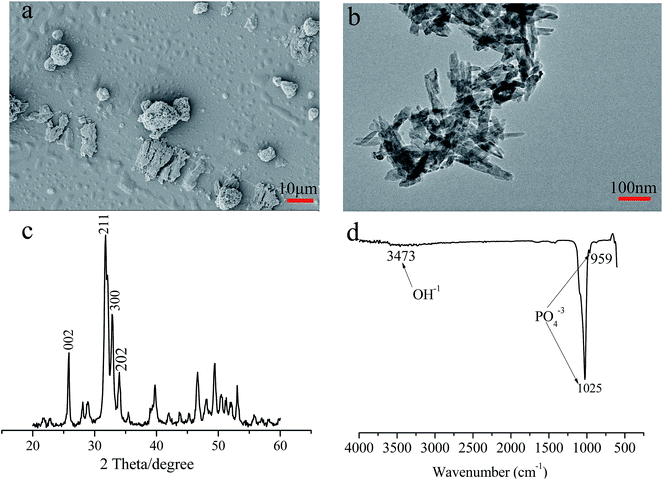
Nano-sized HA particles were fabricated using a water co-precipitation method. Fig. 1a and b shows that the HA particles had a needle-like structure and were typically 100–300 nm long and 20–30 nm in diameter. The XRD pattern of the HA particles is shown in Fig. 1c. The four strongest reflections in the respective powder patterns were indexed as 002, 211, 300 and 202. The FTIR spectrum of the HA particles is shown in Fig. 1d. The characteristic peaks of the HA particles were observed two sharp peaks at 1025 and 959 cm−1 that corresponded to PO43−.3.2. Characterisation of the SF-based scaffolds
FTIR was further used to analyse the molecular conformations of the SF-based scaffolds with or without HA and CNC incorporation. Fig. S3† shows the FTIR spectra of the HA particles, CNC particles, the SF scaffold and the SF-based scaffolds. The SF scaffold exhibited characteristic absorption peaks at 1637 (amide I), 1515 (amide II) and 1237 cm−1 (amide III). CNC exhibited absorption peaks at 1411 and 1014 cm−1 that corresponded to O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations. The CNC/SF scaffold exhibited characteristic peaks that were unique to both the SF scaffold and CNC. The FTIR spectrum of the HA/SF scaffold contained absorption bands that corresponded to both HA particles and the SF scaffold. Additionally, the HA/CNC/SF scaffolds had similar absorption bands as the HA particles, CNC and SF scaffold. These results indicated that HA or CNC incorporation did not affect the molecular conformation of the silk fibroin scaffold.
C stretching vibrations. The CNC/SF scaffold exhibited characteristic peaks that were unique to both the SF scaffold and CNC. The FTIR spectrum of the HA/SF scaffold contained absorption bands that corresponded to both HA particles and the SF scaffold. Additionally, the HA/CNC/SF scaffolds had similar absorption bands as the HA particles, CNC and SF scaffold. These results indicated that HA or CNC incorporation did not affect the molecular conformation of the silk fibroin scaffold.
Fig. 2a shows SEM images of the SF scaffolds. The pore distribution was quite uniform, with an average pore size of 180 ± 7.3 μm (Table 1). Moreover, the porosity of the SF scaffold was 95.2 ± 3.4% with marked interconnectivity. The CNC/SF scaffold (Fig. 2b) had a chaotic porous structure and the CNC were firmly fixed to the walls of the SF structure. The average pore size and porosity of the CNC/SF scaffold were 130 ± 3.5 μm and 94.3 ± 4.5%, respectively (Table 1). Fig. 2c and d shows that the HA/SF and HA/CNC/SF scaffolds had a similar macroporous structure with a pore size of 100–120 μm and a porosity of >90%. The inner pore surface of the HA/SF and HA/CNC/SF scaffolds was densely covered by needle-like HA nanoparticles or CNC/HA composite.
 | ||
| Fig. 2 SEM images of the (a) SF scaffold, (b) CNC/SF scaffold, (c) HA/SF scaffold and (d) HA/CNC/SF scaffold. | ||
| Pore diameter (μm) ± SD | Porosity (%) ± SD | |
|---|---|---|
| SF | 180 ± 7.3 | 95.2 ± 3.4 |
| CNC/SF | 130 ± 3.5 | 94.3 ± 4.5 |
| HA/SF | 104.1 ± 4.9 | 93.3 ± 5.7 |
| HA/CNC/SF | 110.7 ± 7.3 | 90.2 ± 6.2 |
The thermal degradation behaviours of HA, CNC and the SF and SF-based scaffolds are shown in Fig. 3. The TGA curve of pure HA (Fig. 3A) showed that the total mass loss from room temperature to 800 °C was only 12%. This result indicated that the HA material was thermally stable at high temperatures. For the CNC particles and all of the scaffolds, most of the weight loss occurred from water loss at temperatures below 200 °C. From 200–600 °C, the weight loss of the CNC particles and the SF scaffold were greater because of dehydration and decomposition of the macromolecules. For the HA/CNC/SF scaffold, the residual mass rate (%) was lower (ca. 28%) compared with the CNC (ca. 18.6%), CNC/SF scaffold (ca. 26.6%) and SF scaffold (ca. 3.79%), but higher than the HA/SF scaffold (ca. 40.5%).
 | ||
| Fig. 3 Thermogravimetric analyser (TGA) curves of HA, CNC, the SF scaffold and SF-based composite scaffolds. | ||
The mechanical properties of the SF and SF-based scaffolds were determined (Table 2). The compressive stress and modulus of the SF and CNC/SF scaffold were almost identical. However, the compressive stress and modulus were 200.7 ± 15.3 and 617.5 ± 25.2 kPa, respectively, for the HA/CNC/SF scaffold. Furthermore, for HA/SF scaffold, the values were 140.1 ± 11.4 and 428.3 ± 14.4 kPa, respectively.
| Compressive stress (kPa) ± SD | Compressive modulus (kPa) ± SD | |
|---|---|---|
| SF | 92.1 ± 7.3 | 175.2 ± 10.65 |
| CNC/SF | 100.8 ± 13.5 | 200 ± 12.3 |
| HA/SF | 140.1 ± 11.4 | 428.3 ± 14.4 |
| HA/CNC/SF | 200.7 ± 15.3 | 617.5 ± 25.2 |
3.3. Cell viability
The cell viability of the SF and SF-based scaffolds was analysed using the CKK-8 assay (Fig. 4). The viability of the MC3T3-E1 cells in all of the scaffolds increased monotonously with increasing culture time. The proliferation of the MC3T3-E1 cells did not reveal any significant differences when the cells were grown on SF, CNC/SF and HA/SF during the cultivation time. However, compared with the other scaffolds, the cell viability on the HA/CNC/SF scaffold was significantly higher at days 5 and 7, which suggested that the HA/CNC/SF scaffold promoted the proliferation of the MC3T3-E1 cells. The CCK-8 results showed that the relative in vitro cell proliferation abilities on the SF and SF-based scaffolds were as follows: HA/CNC/SF scaffold > HA/SF scaffold > CNC/SF scaffold > SF scaffold. The results indicated that the addition of HA and CNC significantly increased cell proliferation.3.4. ALP activity
Fig. 5 presents the results of the analysis of the ALP activity when the MC3T3-E1 cells were seeded on various scaffolds. Fig. 5 shows that much lower ALP activities were obtained for the groups lacking HA nanoparticles (SF scaffold and CNC/SF scaffold) than for the other groups. This implied that the pure scaffold or the CNC/SF scaffold did not improve the early osteogenesis indicator (ALP activity). In contrast, the other groups (HA/SF scaffold and HA/CNC/SF scaffold) showed significantly improved ALP activities at days 7 and 14. The HA/CNC/SF scaffold exhibited higher ALP activity, which was in good agreement with the cell viability assay. | ||
| Fig. 5 Alkaline phosphatase (ALP) activity assay of MC3T3-E1 cultured on various scaffolds for different time periods. | ||
3.5. In vivo bone regeneration
All of these SF scaffolds were implanted in situ in the calvaria of rats. To measure the new bone formation within the defects, the images of the newly regenerated bone were reconstructed using micro-CT. Fig. 6A shows that after 8 weeks, obvious osteogenesis was observed in the HA/CNC/SF, HA/SF, CNC/SF groups and the defect sizes were also significantly decreased. The HA/CNC/SF group had the minimum volume of remaining bone defect. Additionally, the measured bone mineral density (BMD) (Fig. 6B) also demonstrated that the pure SF scaffolds had a slight effect on defect repair compared with the group with material. Additionally, the BMD values showed that the new bone in the HA/CNC/SF group was significantly greater than in the other groups at 12 weeks. No significant difference was found between the HA/SF and CNC/SF groups.3.6. Histological analysis of bone regeneration
Histomorphometric analyses were conducted to verify the in vivo osteogenic ability of these scaffolds for calvarial defect repair. Fig. 7 shows representative H&E staining that confirmed calvarial defect repair. After 4 weeks implantation, the inflammatory cells were not present around the scaffold and new bone had formed in all of the groups. More new bone formation was observed in the HA/CNC/SF group (Fig. 7D). After 8 weeks, the scaffolds (marked as G) had been significantly degraded but were still detectable. Furthermore, an apparent increase in new bone formation was observed in the HA/SF and HA/CNC/SF groups. Additionally, in the HA/CNC/SF group, many osteoblasts were trapped in the lacunae and the new bone showed a similar morphology to the host bone (Fig. 7H). A small amount of new bone was found in the CNC/SF group (Fig. 7F). Minimal new bone formation was observed in the SF group (Fig. 7E). After 12 weeks, in the SF group (Fig. 7I), fewer new bones had formed compared with those observed in the other groups. In contrast, the CNC/SF, HA/SF and HA/CNC/SF groups (Fig. 7J–L) presented massive bone formation. In particular, the repair effect of the HA/CNC/SF group was significantly better than that of the CNC/SF and HA/SF groups. The scaffolds had significantly degraded but were still visible (marked as J). This result was in good agreement with the micro-CT analysis (Fig. 6A), which showed that the HA and CNC-loaded SF scaffold exhibited the best restoration efficiency among all of the groups. | ||
| Fig. 7 Hematoxylin and eosin (H&E) staining photomicrographs of calvarial defect sites treated with SF (A, E and I), CNC/SF (B, F and J), HA/SF (C, G and K) and HA/CNC/SF (D, H and L). | ||
Safranin O and Fast Green staining was performed after 8 and 12 weeks to further verify the repair effect. Fig. 8 shows that a small amount of new bones (green colour) were observed in CNC/SF, HA/SF and HA/CNC/SF after 4 weeks, except SF group. After 12 weeks, calcified cartilage (red colour) and new bones (green colour) were observed in SF and CNC/SF, while massive new bones (green colour) were found in the HA/SF and HA/CNC/SF groups.
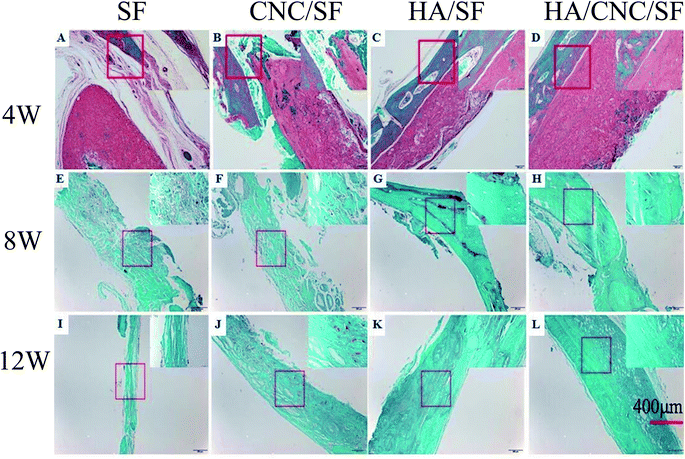 | ||
| Fig. 8 Safranin O and Fast Green stained photomicrographs of calvarial defect sites treated with SF (A, E and I), CNC/SF (B, F and J), HA/SF (C, G and K) and HA/CNC/SF (D, H and L). | ||
4. Discussion
It has been reported that HA displays osteoinductive behaviour during the healing of bone defects because its morphology and crystallinity resemble the inorganic component present in natural bone.27,28 Fig. 1 schematically shows the preparation of the HA nanoparticles by a water co-precipitation method.The synthesis of a composite with HA and CMC via co-precipitation has been reported.29,30 In this study, HA and CNC nanoparticles were uniformly distributed; they were successfully incorporated within the SF scaffolds by freeze-dying an SF solution containing HA and CNC nanoparticles. Compared to the SF scaffold, the pore size and porosity of the HA/CNC/SF scaffold were slightly smaller (110 μm, 90.2%) because of incorporation of the HA and CNC nanoparticles within the SF scaffolds. HA and CNC are powder which will affect the porosity and pore size of SF scaffold. As the HA and/or CNC content increased, the pore size decreased, the similar results have been reported.31 When SF content was high, the internal structure of the scaffold material had laminar pore walls. Furthermore, the porosity and pore size of pure SF scaffolds larger than others, probably because increasing SF content in the system lead to more interaction between fibroin molecules and subsequently larger volume shrinkage effect during the freeze drying process. The thermostability and mechanical properties of the HA/CNC/SF scaffold were significantly improved because of its smaller pore size, uniform pore size distribution and embedding of the HA and CNC nanoparticles in the SF scaffold. Holtorf et al. reported that a scaffold with a porosity of 80% and a pore size of 119 μm was conducive to early osteoblast differentiation.32 Our results suggested that the HA/CNC/SF scaffold might be favourable for bone defect repair.
Biocompatibility is an important criterion for the evaluation of biological scaffolds. Fig. 4 shows that the HA/CNC/SF scaffold had excellent biocompatibility and promoted the proliferation of MC3T3-E1 cells. ALP activity is an indication of bone differentiation and mineralisation. In this study, all four types of scaffolds supported osteogenic differentiation of MC3T3-E1 cells, because the ALP activity gradually increased during the 14 days of culture (Fig. 5). The MC3T3-E1 cells in the HA/CNC/SF group exhibited higher ALP activity compared with the other groups. These results were consistent with their components and physical structures; because the pore size of the HA/CNC/SF scaffold was conducive to early osteoblast differentiation and HA–CNC had osteoinductive behaviour. Pasqui et al. reported that an HA–CNC composite significantly activated TGF-β1, which regulates cell proliferation and is involved in cell metabolism, with different expression according to the microenvironment and cell differentiation stage.33 According to the in vitro results, the HA/CNC/SF scaffolds were highly biocompatible and osteoconductive.
Four types of scaffolds were implanted in rat calvarial defects to repair bone defects. Quantitative micro-CT analysis of the new bone area and BMD in the HA/CNC/SF group showed that they were significant higher than in the other groups (Fig. 6). Furthermore, there was no obvious inflammation reaction in the experiments after 4 weeks of implantation (Fig. 7A–D). That finding demonstrated the excellent biocompatibility of the four types of scaffolds. Moreover, the scaffolds were significantly degraded after 12 weeks of implantation (Fig. 7J), which indicated that the degradation rate of the scaffold was well-matched to the regeneration rate of bone. In general, the scaffold degradation ratio should match the neotissue formation ratio.34 With the degradation of the HA/SF or HA/CNC/SF scaffold, HA could release calcium and phosphate ions, which would stimulate cell chemotaxis and could favour new bone formation in HA/SF or HA/CNC/SF scaffold implants.19 Meanwhile, a HA–CNC composite could significantly activate TGF-β1. Histological staining showed that newly formed bone completely bridged the defect area in the HA/CNC/SF group, whereas the newly formed bone only partly covered the defect area in the SF and CNC/SF groups (Fig. 7 and 8). The in vivo results showed that the HA/CNC/SF scaffold possessed better osteoinductivity, which may have resulted from its promotion of cell proliferation and osteogenic differentiation, and thereby effectively enhanced new bone formation in vivo.
5. Conclusions
Summarising, the results demonstrated the biocompatibility of the HA/CNC/SF scaffold and its potential utility as a scaffold for the efficient repair of critical size rat calvarial defect. The rat calvarial defect healed with new-formed bone within 12 weeks of implantation. Thus, the HA/CNC/SF scaffold may be the ideal candidate for critical size bone defect repair in bone tissue engineering.References
- A. Hokugo, T. Saito, A. Li, K. Sato, Y. Tabata and R. Jarrahy, Biomaterials, 2014, 35, 5565–5571 CrossRef CAS PubMed.
- L. Lin, R. Hao, W. Xiong and J. Zhong, J. Biosci. Bioeng., 2015, 119, 591–595 CrossRef CAS PubMed.
- H. Nie, M. L. Ho, C. K. Wang, C. H. Wang and Y. C. Fu, Biomaterials, 2009, 30, 892–901 CrossRef CAS PubMed.
- B. R. Constantz, I. C. Ison, M. T. Fulmer, R. D. Poser, S. T. Smith, M. VanWagoner, J. Ross, S. A. Goldstein, J. B. Jupiter and D. I. Rosenthal, Science, 1995, 267, 1796–1799 CAS.
- H. Petite, V. Viateau, W. Bensaid, A. Meunier, C. de Pollak, M. Bourguignon, K. Oudina, L. Sedel and G. Guillemin, Nat. Biotechnol., 2000, 18, 959–963 CrossRef CAS PubMed.
- C. Delloye, O. Cornu, V. Druez and O. Barbier, J. Bone Jt. Surg., Br. Vol., 2007, 89, 574–579 CrossRef CAS PubMed.
- K. Partridge, X. Yang, N. M. P. Clarke, Y. Okubo, K. Bessho, W. Sebald, S. M. Howdle, K. M. Shakesheff and R. O. C. Oreffo, Biochem. Biophys. Res. Commun., 2002, 292, 144–152 CrossRef CAS PubMed.
- D. Li, C. Ye, Y. Zhu, Z. Gou and C. Gao, J. Biomed. Mater. Res., Part B, 2012, 100, 1103–1113 CrossRef PubMed.
- L. Li, G. Zhou, Y. Wang, G. Yang, S. Ding and S. Zhou, Biomaterials, 2015, 37, 218–229 CrossRef CAS PubMed.
- M. M. Stevens, Mater. Today, 2008, 11, 18–25 CrossRef CAS.
- D. W. Hutmacher, Biomaterials, 2000, 21, 2529–2543 CrossRef CAS PubMed.
- H. Liu, G. W. Xu, Y. F. Wang, H. S. Zhao, S. Xiong, Y. Wu, B. C. Heng, C. R. An, G. H. Zhu and D. H. Xie, Biomaterials, 2015, 49, 103–112 CrossRef CAS PubMed.
- K. L. Lin, C. T. Wu and J. Chang, Acta Biomater., 2014, 10, 4071–4102 CrossRef CAS PubMed.
- U. Ripamonti, Biomaterials, 1996, 17, 31–35 CrossRef CAS PubMed.
- H.-W. Kim, H.-E. Kim and V. Salih, Biomaterials, 2005, 26, 5221–5230 CrossRef CAS PubMed.
- C. Chen, H. Li, J. Pan, Z. Yan, Z. Yao, W. Fan and C. Guo, Biotechnol. Lett., 2015, 37, 457–465 CrossRef CAS PubMed.
- E. Wenk, A. J. Meinel, S. Wildy, H. P. Merkle and L. Meinel, Biomaterials, 2009, 30, 2571–2581 CrossRef CAS PubMed.
- N. Kasoju and U. Bora, J. Biomed. Mater. Res., Part B, 2012, 100, 1854–1866 CrossRef PubMed.
- K. Wei, et al., Fabrication of nano-hydroxyapatite on electrospun silk fibroin nanofiber and their effects in osteoblastic behavior, J. Biomed. Mater. Res., Part A, 2011, 97(3), 272–280 CrossRef PubMed.
- X. Ren, V. Tu, D. Bischoff, D. W. Weisgerber, M. S. Lewis, D. T. Yamaguchi, T. A. Miller, B. A. Harley and J. C. Lee, Biomaterials, 2016, 89, 67–78 CrossRef CAS PubMed.
- Y. X. Pang and X. Bao, J. Eur. Ceram. Soc., 2003, 23, 1697–1704 CrossRef CAS.
- J. Kundu, M. Dewan, S. Ghoshal and S. C. Kundu, J. Mater. Sci.: Mater. Med., 2008, 19, 2679–2689 CrossRef CAS PubMed.
- P. Wongpanit, H. Ueda, Y. Tabata and R. Rujiravanit, J. Biomater. Sci., Polym. Ed., 2010, 21, 1403–1419 CrossRef CAS PubMed.
- H. She, X. Xiao and R. Liu, J. Mater. Sci., 2007, 42, 8113–8119 CrossRef CAS.
- Z. Yin, X. Chen, J. L. Chen, W. L. Shen, T. M. Hieu Nguyen, L. Gao and H. W. Ouyang, Biomaterials, 2010, 31, 2163–2175 CrossRef CAS PubMed.
- D. Li, W. Wang, R. Guo, Y. Qi, Z. Gou and C. Gao, Chin. Sci. Bull., 2011, 57, 435–444 CrossRef.
- J. Liuyun, L. Yubao and X. Chengdong, J. Mater. Sci.: Mater. Med., 2009, 20, 1645–1652 CrossRef PubMed.
- J. Xu, K. A. Khor, Z. Dong, Y. Gu, R. Kumar and P. Cheang, Mater. Sci. Eng., A, 2004, 374, 101–108 CrossRef.
- N. Zakharov, Z. A. Ezhova, V. Kalinnikov and A. Chalykh, Inorg. Mater., 2005, 41, 509–515 CrossRef CAS.
- N. A. Peppas, Hydrogels in medicine and pharmacy, CRC Press, Boca Raton, Fla, 1986 Search PubMed.
- S. Zeng, L. Liu, Y. Shi, J. Qiu, W. Fang, M. Rong, Z. Guo and W. Gao, PLoS One, 2015, 10, e0128658 Search PubMed.
- H. L. Holtorf, N. Datta, J. A. Jansen and A. G. Mikos, J. Biomed. Mater. Res., Part A, 2005, 74, 171–180 CrossRef PubMed.
- D. Pasqui, P. Torricelli, M. De Cagna, M. Fini and R. Barbucci, J. Biomed. Mater. Res., Part A, 2014, 102, 1568–1579 CrossRef PubMed.
- Y. Lan, W. Li, Y. Jiao, R. Guo, Y. Zhang and W. Xue, Acta Biomater., 2014, 10, 3167–3176 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra02038k |
| This journal is © The Royal Society of Chemistry 2016 |